Heat Engines
- A heat engine is a device that converts thermal energy into mechanical work
- Heat engines operate through a series of thermodynamic processes which form a closed cycle
- A closed cycle is one in which the system returns to its initial state
- A simple heat engine consists of a gas in a cylinder with a piston

A simple heat engine converts thermal energy into mechanical work
- The steps in the operation of a cyclic heat engine process are:
1. Extract heat from a hot reservoir
-
- A hot reservoir (a source of thermal energy) at a high temperature
transfers heat
into the engine
- A hot reservoir (a source of thermal energy) at a high temperature
2. Use some of the extracted heat to perform work
-
- The gas does mechanical work as it expands which pushes the piston out
3. Release excess heat into a cold reservoir
-
- The gas is allowed to cool at constant volume, meanwhile, heat
is released to the surroundings
- Some of the energy transferred into the engine is released into a cold reservoir (a sink for excess heat) at a lower temperature
- The gas is allowed to cool at constant volume, meanwhile, heat
4. Repeat cycle
-
- Once the heat has been extracted, the piston is pushed down to compress the gas back to its original state
- The process can then be repeated as many times as needed, continuously converting heat into mechanical work

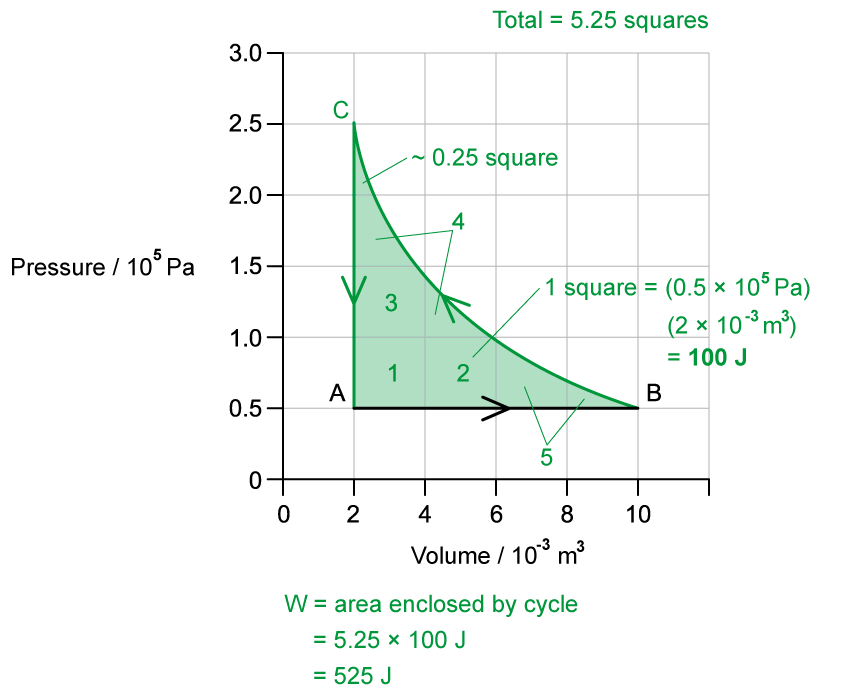
- For a cyclic heat engine process, the p-V diagram will form a closed loop
- The area inside the loop is equal to the net work done during one cycle

- The net work done by the engine is:
- Where:
= useful work output of the heat engine (J)
= heat transferred from hot reservoir to engine (J)
= heat transferred from engine to cold reservoir (J)