Titration Curves of Polyprotic Acids
Polyprotic Acids
- Polyprotic acids contain more than one ionising hydrogen atoms
- E.g. H2SO4, H3PO4
- These acids ionize and stepwise
Ionization steps for phosphoric acid, H3PO4
| Step | Equation |
| Step 1 | H3PO4 |
| Step 2 | H2PO4– |
| Step 3 | HPO4– |
- Titrations of polyprotic acids have more than one equivalence point due to the series of ionization steps
- If H3PO4 is titrated against a strong base such as NaOH
- At each equivalence point, the acid is neutralised to H2PO4, HPO42– and PO43– respectively
- The pH at these points can be determined by using
- Ka =
- Ka =
- Between the equivalence points, there are mixtures of a conjugate acid and its conjugate base and are known as buffer regions
- At the midpoint of these regions, the pH = pKa of the acid and the concentration of the conjugate acid and base are equal
- E.g. [H2PO4] = [HPO42–]
- This is the half-equivalence point
pH curve for addition of NaOH to H3PO4
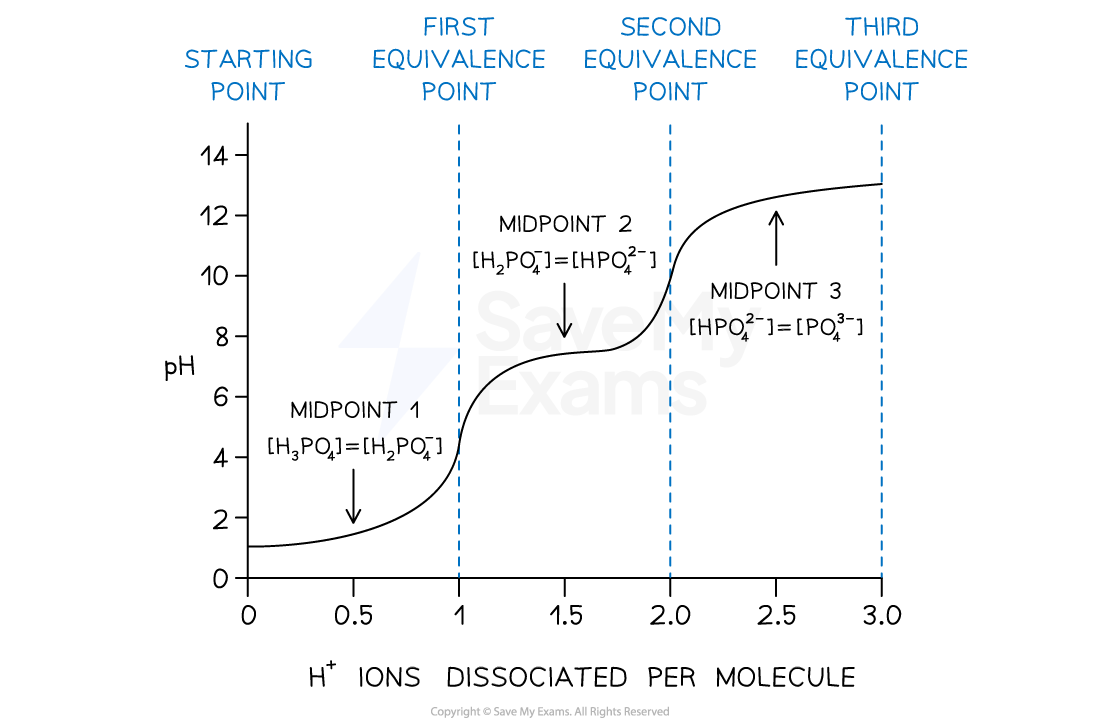
At the midpoints of each ionization step, the pH of the acid is equal to the pKa

