Hydrogen Bonding
- This is a special type of dipole-dipole interaction found in covalent molecules in which hydrogen is directly bonded to either a nitrogen, oxygen or fluorine atom
- These elements are very electronegative
- Therefore, they form polar bonds with hydrogen
Polar covalent bond H—F bond
A polar covalent bond in hydrogen fluoride is described by the presence of δ+ on the hydrogen and δ- charges on the electronegative fluorine atom
- The hydrogen atom is significantly less electronegative than a nitrogen, oxygen or fluorine atom
- Therefore, the hydrogen atom has a partial positive (δ+) charge
- The very electronegative element has a partial negative charge (δ-)
- Hydrogen bonding describes the attraction between the δ+ hydrogen atom (Hδ+) in this polar bond and the nonbonding electron pairs on a nearby small electronegative atom or ion
- Compared to dipole-dipole or London dispersion forces, hydrogen bonds are a stronger intermolecular force
Hydrogen Bonding
Hydrogen bonding between molecules of ammonia, water and hydrogen fluoride
- The strength of the hydrogen bond is highest in molecules where the hydrogen atom is bonded to a fluorine atom
- This is because fluorine has the highest electronegativity
- Generally, the strength of hydrogen bonds increases in the order:
H—N < H—O < H—F
- However, the strength of hydrogen bond in a molecule is also dependent on how many hydrogen bonds can be formed per pair of molecules
- For example, acetic acid, CH3COOH, and propanol, CH3CH2CH2OH:
- They both have the same molecular mass, 60 amu
- They are both capable of forming hydrogen bonds
- However, a pair of acetic acid molecules can form two hydrogen bonds, whereas a pair of propanol molecules can form only one
- Hence, the boiling point of acetic acid is greater than propanol
Multiple Hydrogen Bonds
Hydrogen bonding in acetic acid and propanol. Each acetic acid can form two hydrogen bonds while propanol can form only one hydrogen bond.
- Similarly, the hydrogen bonds in water are greater than that in hydrogen fluoride.
- Water can form two hydrogen bonds
- This is because there are 2 lone pairs of electrons on the oxygen atom AND two available δ+ hydrogen atoms
- Hydrogen fluoride forms only one hydrogen bond
- This is because, although there are 3 lone pairs of electrons on the fluorine atom, there is only one available δ+ hydrogen atom
- Hence water has a higher boiling point than hydrogen fluoride
- Water can form two hydrogen bonds
Hydrogen Bonding in H2O and HF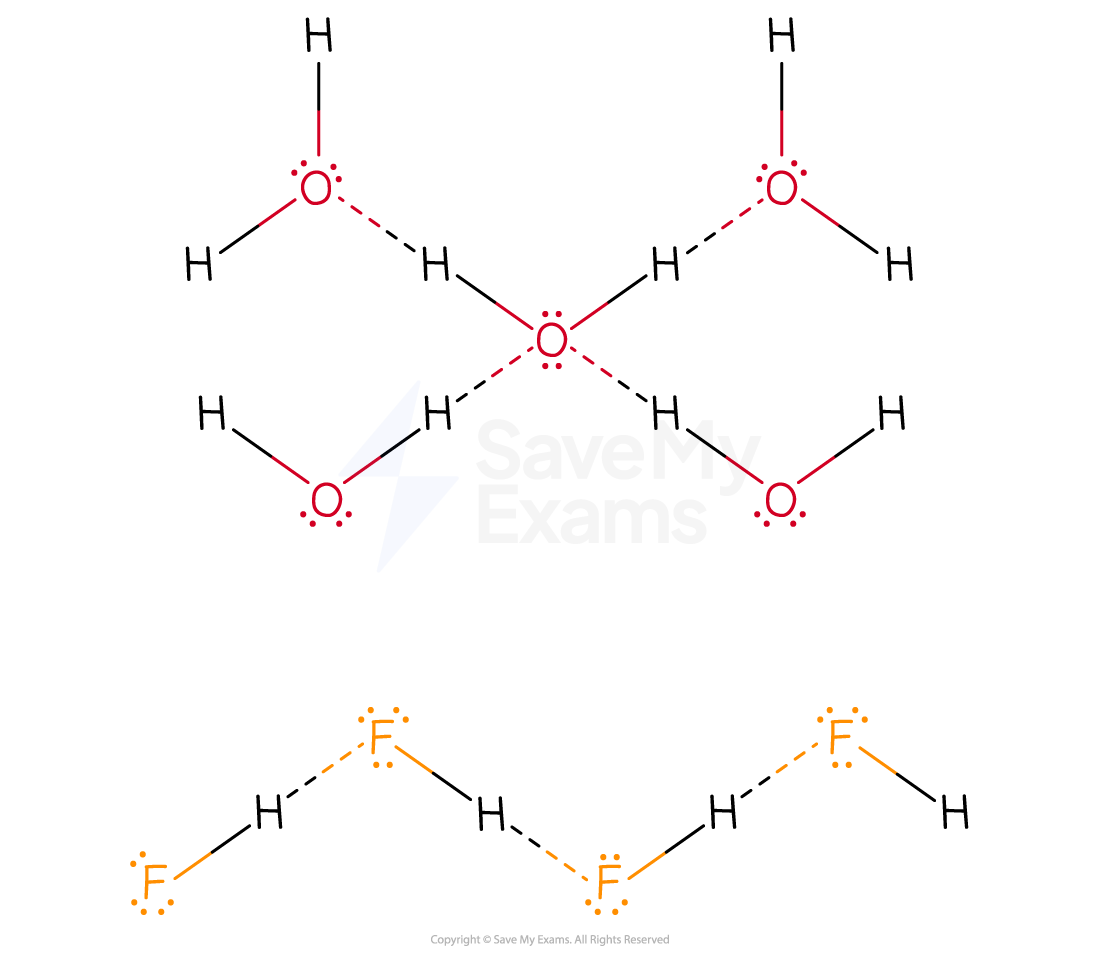
The diagram shows two hydrogen bonds per pair of water molecules compared to only one per pair of hydrogen fluoride
Exam Tip
- A covalent molecule may not be able to form hydrogen bonds with itself due to the lack of a polar hydrogen atom yet forms hydrogen bonds with another molecule with a polar hydrogen atom
- For example, acetaldehyde, CH3CHO, can form hydrogen bonds with water molecules which makes it soluble in water

