Physical & Chemical Changes (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Physical & chemical changes
Physical changes
Physical changes do not produce any new chemical substances
These changes are often:
Easy to reverse
Relatively easy to separate
Examples of physical changes include:
Changing state, e.g. melting / solid → liquid
Making a mixture from 2 or more substances
Dissolving a solute in a solvent
Chemical changes
During chemical changes / reactions, new chemical substances are formed that have very different properties to the reactants
Most chemical changes are difficult to reverse
There may be signs that a new substance has formed, such as:
Colour changes
Temperature changes
Effervescence (fizzing)
Colour change
One example of a reaction that shows a colour change is the metal displacement reaction of silver and copper
Orange-brown copper metal is added to a colourless solution of silver nitrate
As the reaction proceeds the copper displaces the silver from the solution
This causes two colour changes:
The solid inside the beaker changes from orange-brown to silver
The solution changes from colourless to blue
The metal displacement reaction of silver and copper
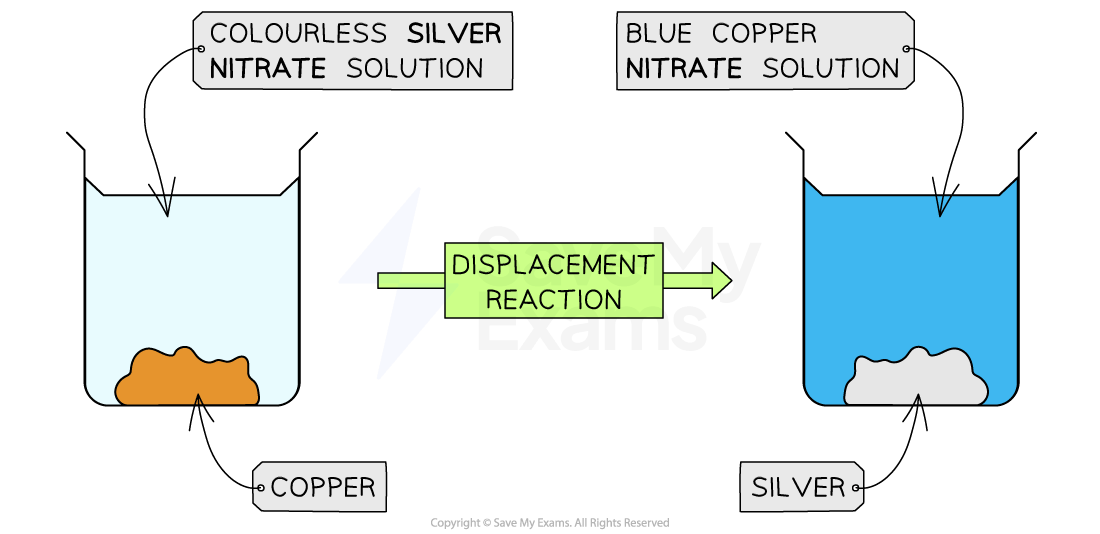
The reaction shows two colour changes; one in the colour of the solution and one in the colour of the solid
Another reaction, from the syllabus, that shows a clear colour change is the halide ion test
From colourless to cream / white / yellow depending on the halide ion
This also includes a change of state as a solid / precipitate is formed
Temperature change
Chemical reactions that give a temperature change can be grouped into two categories:
Those that give out heat / thermal energy - exothermic
Those that take in heat / thermal energy - endothermic
Exothermic reactions
Some examples of reactions that cause an increase in temperature are:
The reaction of calcium oxide with water to form calcium hydroxide is highly exothermic
The reaction of sodium with water is exothermic and effervesces
Endothermic reactions
Reactions that cause a decrease in temperature are less commonly talked about than exothermic reactions
Examples include:
Photosynthesis
Light energy is absorbed during the process of converting carbon dioxide and water into glucose and oxygen
When solid ammonium chloride is dissolved in water
Heat / thermal energy is absorbed from the surroundings, causing the temperature to decrease
This reaction is commonly used in cold packs
Effervescence
Effervescence, or fizzing, is another sign of a chemical reaction
Chemical reactions that cause effervescence often involve acids:
The reaction of alkali metals with water
The reaction of the alkali metals, such as sodium, with water releases hydrogen gas
Metal + water → metal hydroxide + hydrogen
There are other signs of a chemical reaction including:
Light being produced
A smell being produced
A change in pH

Unlock more, it's free!
Did this page help you?