Specific Heat & Latent Heat (Edexcel GCSE Combined Science): Revision Note
Exam code: 1SC0
Specific Heat & Latent Heat
Specific Heat Capacity
If the temperature of the system increases, the increase in temperature of this system depends on:
The mass of the substance heated
The type of material
The energy input to the system
The specific heat capacity of a substance is defined as:
The amount of energy required to raise the temperature of 1 kg of the substance by 1 °C
Different substances have different specific heat capacities
If a substance has a low specific heat capacity, it heats up and cools down quickly (ie. it takes less energy to change its temperature)
If a substance has a high specific heat capacity, it heats up and cools down slowly (ie. it takes more energy to change its temperature)
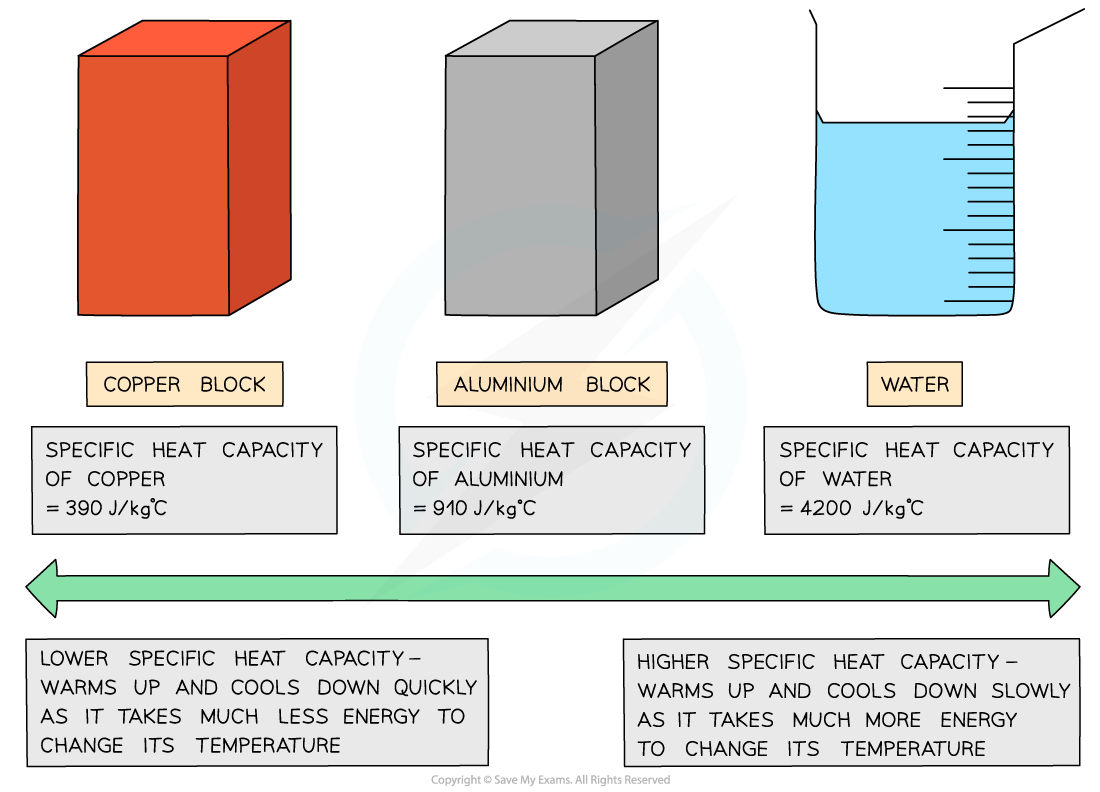
Low v high specific heat capacity
Specific heat capacity is mainly used for liquids and solids
The specific heat capacity of different substances determines how useful they would be for a specific purpose eg. choosing the best material for kitchen appliances
Good electrical conductors, such as copper and lead, are excellent conductors of heat due to their low specific heat capacity
On the other hand, water has a very high specific heat capacity, making it ideal for heating homes as the water remains hot in a radiator for a long time
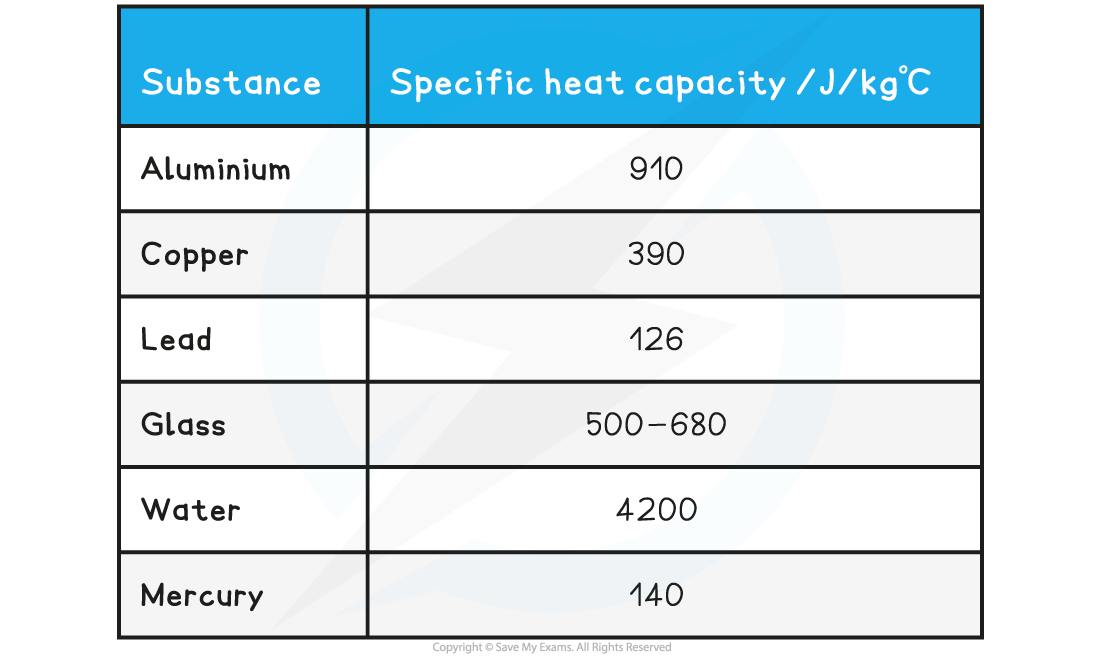
The specific heat capacity of some substances are given in the table below as examples:
Table of values of specific heat capacity for various substances
Specific Latent Heat
Energy is required to change the state of a substance
This energy is known as latent heat
The specific latent heat is defined as:
The amount of thermal energy required to change the state of 1 kg of a substance with no change in temperature
There are two types of specific latent heat:
Specific latent heat of fusion (solid to liquid and vice versa)
Specific latent heat of vaporisation (liquid to gas and vice versa)
Latent heat is represented by the symbol L with units joules per kilogram (J/kg)
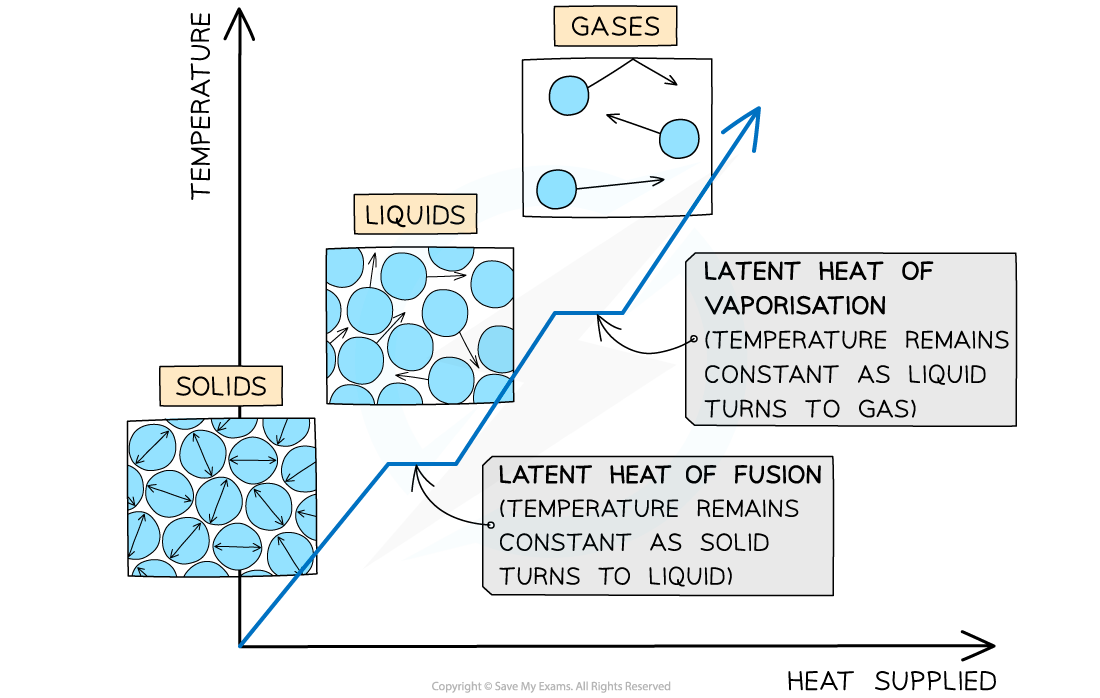
The changes of state with heat supplied against temperature
The specific latent heat of fusion is defined as:
The thermal energy required to convert 1 kg of solid to liquid with no change in temperature
This is used when melting a solid or freezing a liquid
When a solid substance is melted, its temperature stays constant until all of the substance has melted
The latent heat of fusion is the energy needed to break the bonds between the molecules
The specific latent heat of vaporisation is defined as:
The thermal energy required to convert 1 kg of liquid to gas with no change in temperature
This is used when vaporising a liquid or condensing a gas
When a liquid substance is heated, at its boiling point, the substance boils and turns into vapour
The latent heat of vaporisation is the energy needed by the particles to break away from their neighbouring particles in the liquid
Specific heat capacity and specific latent heat are slightly different
Specific heat capacity is used for a change in temperature in the same state
Specific latent heat is used for a change in state but no change in temperature

Difference between specific latent heat and specific heat capacity
Examiner Tips and Tricks
The specific latent heat of fusion and vaporisation value of all substances will be provided for you in the exam question, so you do not need to memorise the value of any. However, make sure to include 'with no change in temperature' in your definition of specific latent heat to be awarded full marks. Use these reminders to help you remember which type of latent heat is being referred to:
Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas

Unlock more, it's free!
Did this page help you?