Pressure & Volume (AQA GCSE Physics): Revision Note
Exam code: 8463
Pressure Changes in a Gas
If the temperature of a gas remains constant, the pressure of the gas changes when it is:
Compressed – decreases the volume which increases the pressure
Expanded – increases the volume which decreases the pressure

Pressure increases when a gas is compressed
The pressure produces a net force at right angles to the wall of the gas container (or any surface)

Gas molecules bouncing off the walls of a container
Therefore, if the gas is compressed, the molecules will hit the walls of the container more frequently
This creates a larger overall net force on the walls which increases the pressure
Did this video help you?
Pressure & Volume
In a gas, the molecles are widely spread
This makes the gas easy to expand and compress
Changing the pressure acting on the gas will compress it or allow it to expand if the temperature is kept constant
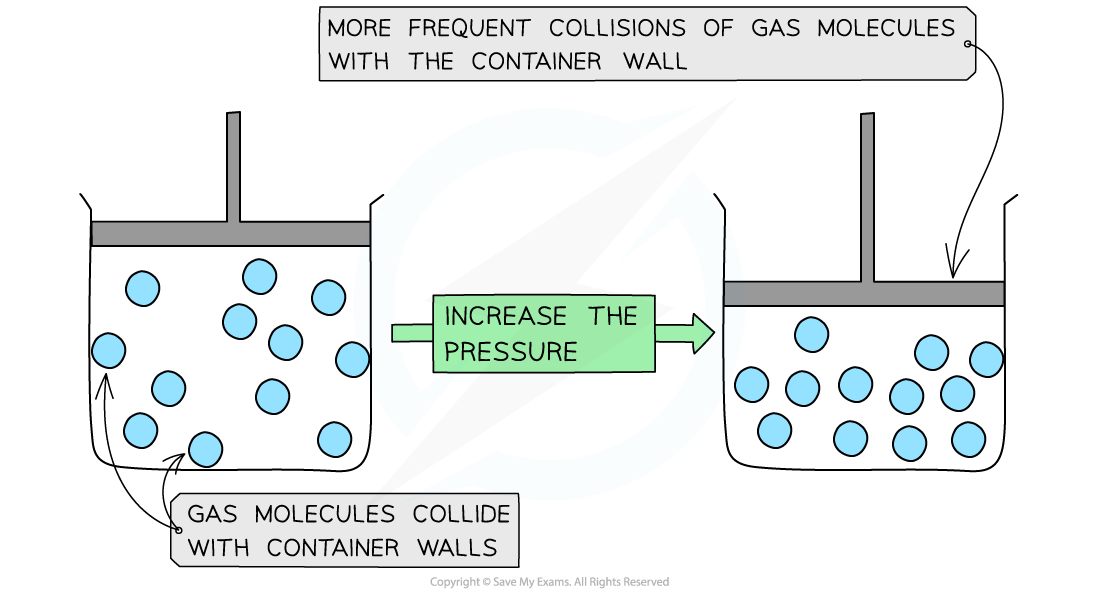
When a gas is compressed, the volume is decreased
The density of the gas increases, allowing more frequent collisions of the molecules on the container wall
This increases the pressure
Calculating Change in Pressure & Volume
For a fixed mass of a gas held at a constant temperature:
pV = constant
Where:
p = pressure in pascals (Pa)
V = volume in metres cubed (m3)
This means that the pressure and volume are inversely proportional to each other
When the volume decreases (compression), the pressure increases
When the volume increases (expansion), the pressure decreases
This is because when the volume decreases, the same number of particles collide with the walls of a container but more frequently as there is less space
However, the particles still collide with the same amount of force meaning greater force per unit area (pressure)
The key assumption is that the temperature and the mass (and number) of the particles remains the same
This equation can also be rewritten for comparing the pressure and volume before and after a change in a gas:
P1V1 = P2V2
Where:
P1 = initial pressure in pascals (Pa)
V1 = initial volume in metres cubed (m3)
P2 = final pressure in pascals (Pa)
V2 = final volume in metres cubed (m3)
This equation is sometimes referred to as Boyle's Law

Initial pressure and volume, P1 and V1, and final pressure and volume, P2 and V2
Worked Example
A gas occupies a volume of 0.70 m³ at a pressure of 200 Pa. Calculate the pressure exerted by the gas if it is compressed to a volume of 0.15 m³.Assume that the temperature and mass of the gas stay the same.
Answer:

Examiner Tips and Tricks
Always check whether your final answer makes sense. If the gas has been compressed, the final pressure is expected to be more than the initial pressure (like in the worked example). If this is not the case, double-check the rearranging of any formulae and the values put into your calculator.
It is very easy for students to overlook the key assumptions of Boyle's law; that (a) the mass, and therefore the number of particles remains the same, and that (b) the temperature remains unchanged. This is a really important piece of information that often comes up in exam questions. Remember that if the mass or temperature is changed for any reason, then Boyle's law will no longer apply.

Unlock more, it's free!
Did this page help you?