DNA Structure (DP IB Biology): Revision Note
Significance of Directionality
Directionality of RNA and DNA
When nucleotides are linked together to form nucleic acids, such as RNA and DNA, the phosphate groups form a bridge between carbon-3 of one sugar molecule and carbon-5 of the next one
This means that each polynucleotide strand has a 3' end where the OH group is located on carbon-3 of the sugar molecule and a 5' end containing the phosphate group on carbon-5
In a DNA molecule, one strand runs from 5' to 3' while the other strand runs from 3' to 5'
This is why the two strands are said to be antiparallel
The directionality of polynucleotide strands plays an important role in the processes of:
DNA replication
Transcription
Translation
During transcription, the genetic code on one of the DNA strands (the coding strand) is transcribed into a strand of mRNA
The coding strand of DNA has the same sequence as the mRNA (except T is replaced with U)
During transcription, the template strand is read in the 3' to 5' direction to synthesise mRNA in the 5' to 3' direction
The mRNA will move into the cytoplasm of the cell, where ribosomes will translate the transcribed code in the 5' to 3' direction
The base sequence of the genetic code will determine the specific order of the amino acids in the polypeptide chain created during translation
Directionality in RNA and DNA are therefore crucially important to ensure that the genetic code is copied, transcribed and translated correctly
DNA Helix Structure
Purine to pyrimidine bonding in the DNA helix structure
Francis Crick and James Watson were two Cambridge scientists who worked together to establish the double helix structure of DNA in 1953
Through trial and error, they managed to build a model of the DNA double helix structure where the different base pairs fit together correctly
The base pairings A to T and C to G are equal in length, meaning that the DNA helix will have the same 3D structure regardless of the base sequence
Adenine (A) and guanine (G) are purine bases while thymine (T) and cytosine (C) are pyrimidine bases
Purines are larger in size than pyrimidines due to their two carbon ringed structure
The stability of the double helix is further increased by the hydrogen bonds that form between these complementary base pairs
Purines and pyrimidines diagram

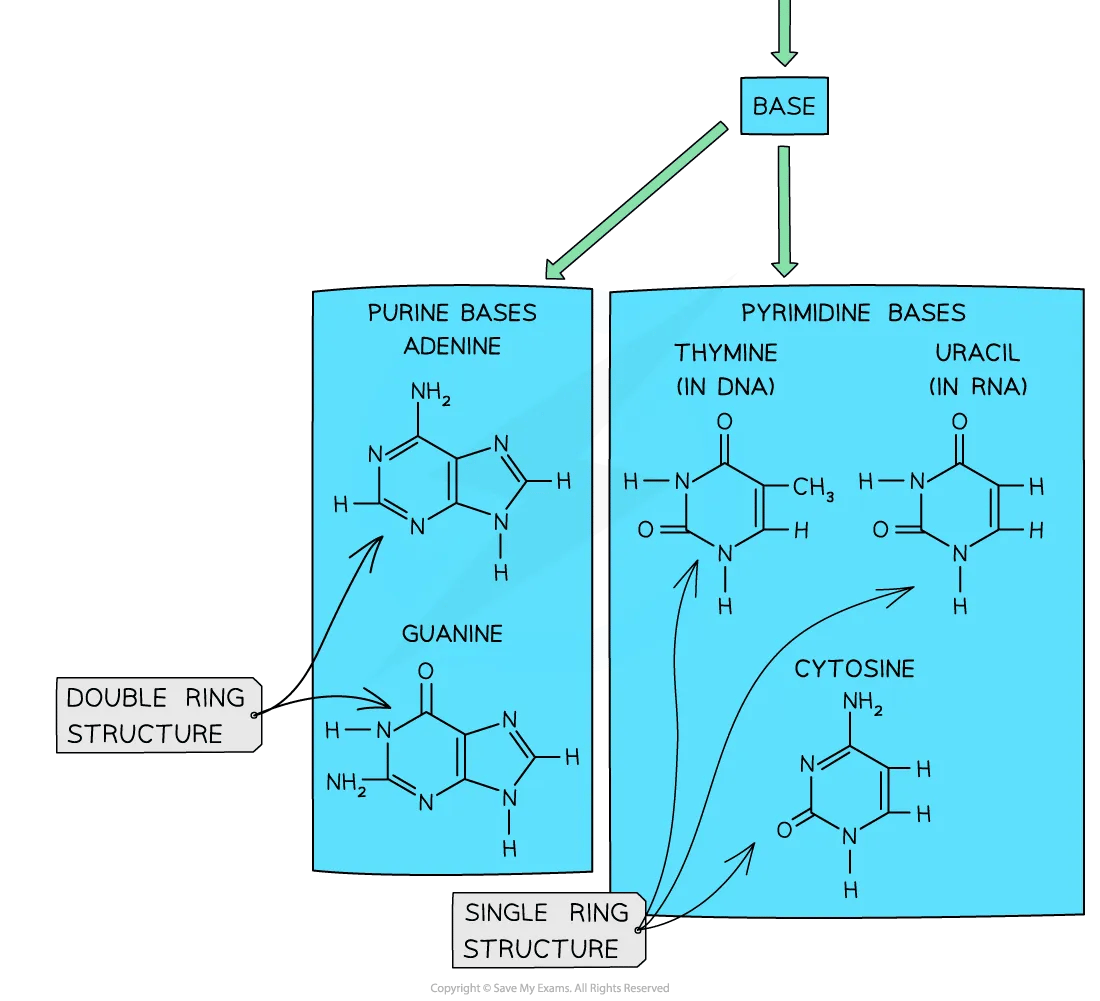
The different sizes of purine and pyrimidine bases mean that they can only pair up in a very specific way. Note that you do not need to know the structural formulae of purines and pyrimidines

Unlock more, it's free!
Did this page help you?