The Photon (OCR AS Physics): Revision Note
Exam code: H156
The Photon Model
Light waves can behave like particles (i.e. photons) and waves
This phenomenon is called the wave-particle nature of light or wave-particle duality
Light interacts with matter, such as electrons, as a particle
The evidence for this is provided by the photoelectric effect
Light propagates through space as a wave
The evidence for this comes from the diffraction and interference of light in Young’s Double Slit experiment
Light as a Particle
The photon model of light explains that:
Electromagnetic waves carry energy in discrete packets called photons
The energy of the photons are quantised according to the equation E = hf
In the photoelectric effect, each electron can absorb only a single photon - this means only the frequencies of light above the threshold frequency will emit a photoelectron
Although the wave theory provided good explanations for phenomena such as interference and diffraction, it failed to explain the photoelectric effect

Defining the Photon
Photons are fundamental particles which make up all forms of electromagnetic radiation
A photon is defined as:
A massless “packet” or a “quantum” of electromagnetic energy
This means that the energy is not transferred continuously but as discrete packets of energy
In other words, each photon carries a specific amount of energy, and transfers this energy all in one go, rather than supplying a consistent amount of energy
Examiner Tips and Tricks
Make sure you learn the definition for a photon: discrete quantity / packet / quantum of electromagnetic energy are all acceptable definitions.
Energy of a Photon
The energy of a photon can be calculated using the formula:
E = hf
Using the wave equation, energy can also be equal to:

Where:
E = energy of the photon (J)
h = Planck's constant (J s)
c = the speed of light (m s-1)
f = frequency (Hz)
λ = wavelength (m)
This equation shows:
The higher the frequency of EM radiation, the higher the energy of the photon
The energy of a photon is inversely proportional to the wavelength
A long-wavelength photon of light has a lower energy than a shorter-wavelength photon
Worked Example
Light of wavelength 490 nm is incident normally on a surface, as shown in the diagram.
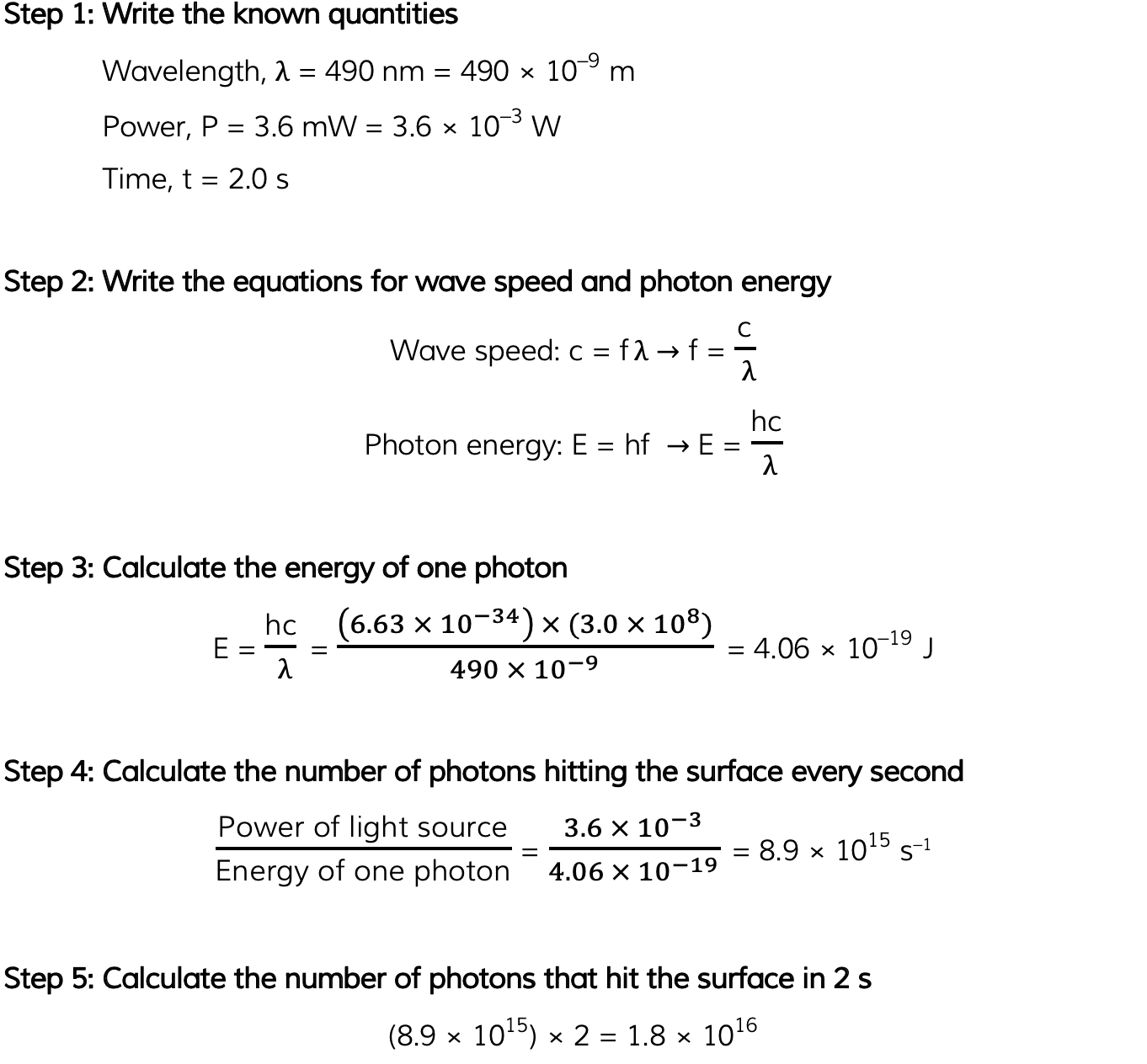
The power of the light is 3.6 mW. The light is completely absorbed by the surface. Calculate the number of photons incident on the surface in 2.0 s.
Answer:
Examiner Tips and Tricks
The values of Planck’s constant and the speed of light will always be available on the datasheet, however, it helps to memorise them to speed up calculation questions!Since Planck's constant is in J s, you may need to convert from eV to J and vice versa for the energy

Unlock more, it's free!
Did this page help you?