Collision Model (College Board AP® Chemistry): Study Guide
Molecular Collisions
On a molecular level, the effect of temperature and concentration can be better understood by the collision model
The key idea of the collision model is that molecules must collide to react
Basically, the greater the number of collisions between molecules per second, the faster the reaction
Hence, when the concentration of reactants increases, the number of collisions increases resulting in an increased rate of reaction
According to the kinetic-molecular theory, increasing the temperature increases the average kinetic energy of the molecules
This makes the molecules move faster, colliding more frequently and with more energy which then means a faster reaction rate
An increase in the surface area of solid reactants also increases the reaction rate
As a result of the increase in the number of contact points for collision and the consequent increase in the frequency of collision
However, it is important to note that not every collision between reactant molecules results in product formation
Collisions which result in reactions are said to be effective collisions and possess two characteristics:
Reacting molecules must collide with sufficient energy greater or equal to some minimum energy known as the activation energy (kinetic energy factor)
Reacting molecules are properly oriented towards each other (orientation factor)
Orientation Factor
The relative orientations of the molecules during collision determine whether the atoms are suitably positioned to form new bonds
Essentially, molecules have to be in the correct position to react
Consider the reaction between CO and NO2 to form CO2 and NO:
The reaction only happens when the carbon atom of the CO molecule strikes an oxygen atom of the NO2 molecule
Orientation Factor and Effective Collision
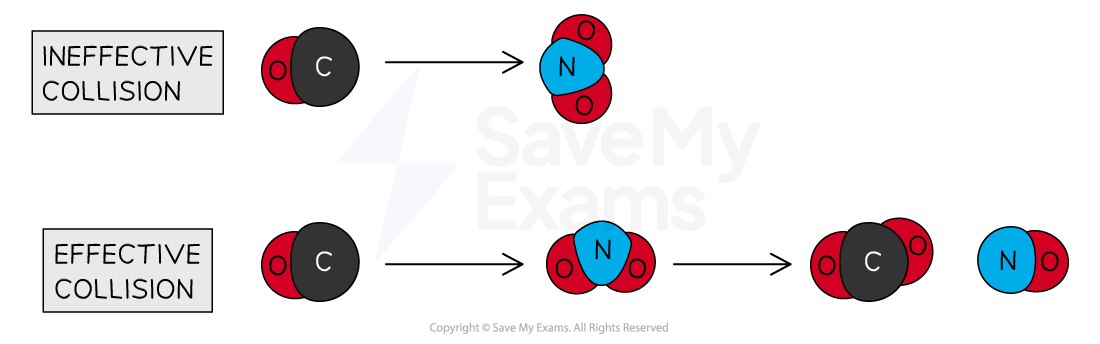
Diagram showing the effect of proper orientation of colliding molecules for a successful reaction. In the reaction between CO and NO2, a successful collision is obtained when the carbon atom of the CO strikes the oxygen atom of the NO2.
According to the collision model, the rate constant, k, which indicates the rate of a reaction, is a product of three factors:
Z, the collision frequency, which gives the number of molecular collisions occurring in unit time at unit concentrations of reactants
f, the fraction of collisions having energy greater than the activation energy
p, the steric factor, which accounts for the fraction of collisions that occur with the reactant molecules properly oriented
With this, the collision model can be made quantitative with the expression:
k = p × Z × f
Worked Example
Based on the collision model, which of the following statements explains the energy requirements of a reaction?
The rate of reaction increases with both increasing temperature and activation energy
The rate of reaction increases with both decreasing temperature and activation energy
The rate of reaction increases with both increasing temperature and decreasing activation energy
The rate of reaction increases with both decreasing temperature and increasing activation energy
Answer:
Option C is correct because:
An increase in temperature increases the average kinetic energy of the reacting particles leading to a faster reaction
A decrease in activation energy increases the fraction of molecules with effective collisions leading to a faster reaction
Maxwell-Boltzmann Distribution Curves
One very important factor that brings about a high number of effective collisions is the kinetic energy of reactant molecules
Reactant molecules are held together by strong chemical bonds
When molecules collide, they must be moving rapidly enough to supply the required energy to break these bonds
Molecules with small kinetic energies bounce off one another without a reaction
Kinetic Energy and Effective Collision
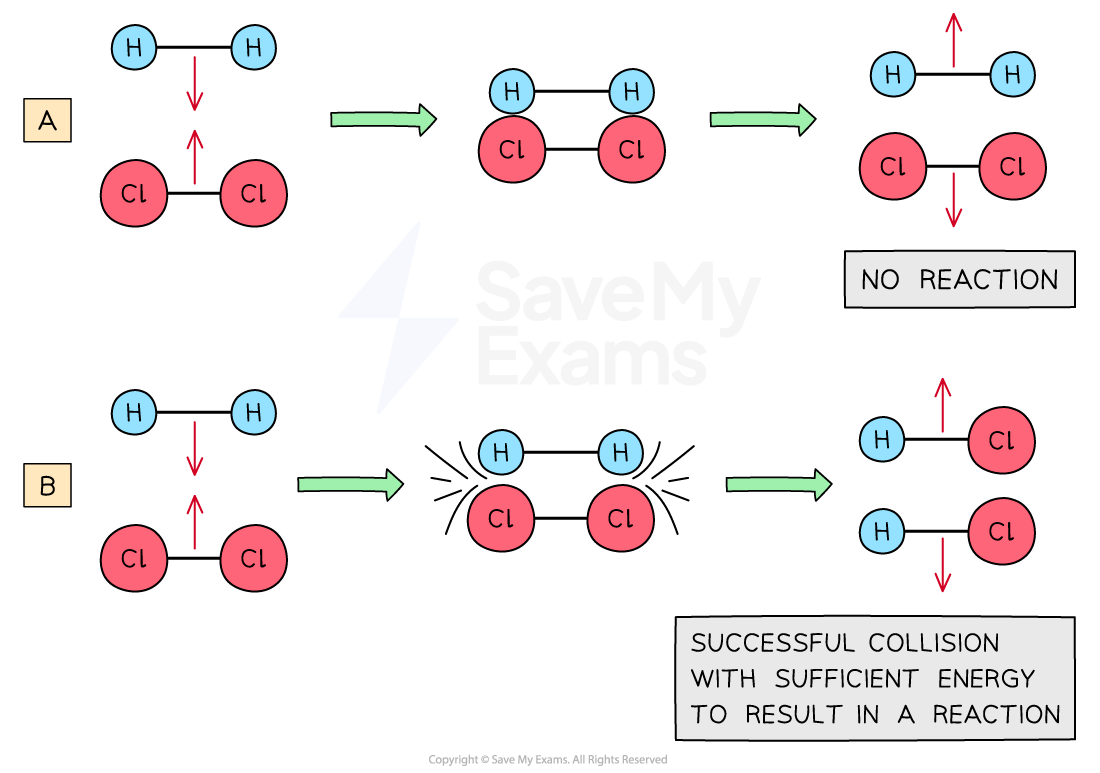
Diagram (A) shows an ineffective collision due to the particles not having enough energy whereas (B) shows an effective collision where the particles have the correct orientation and enough energy for a chemical reaction to take place
The minimum energy which the reactant molecules must produce on collision in order to initiate the reaction is known as the activation energy, Ea
It is a positive quantity, measured in kJ/mol
Maxwell-Boltzmann Distribution, Temperature and Activation Energy
The effect of temperature on reaction rate can be explained using the kinetic molecular theory
Remember: An increase in temperature increases the fraction of molecules with high velocity and hence high kinetic energy
A higher temperature means a larger fraction of molecules that can provide the activation energy required for the reaction
The relationship between the energies of colliding molecules and the activation energy required for a reaction at a certain temperature is described by a Maxwell-Boltzmann distribution curve
This curve can be used to qualitatively estimate the fraction of molecules with sufficient energy that results in a reaction
Maxwell-Boltzmann Distribution Curve
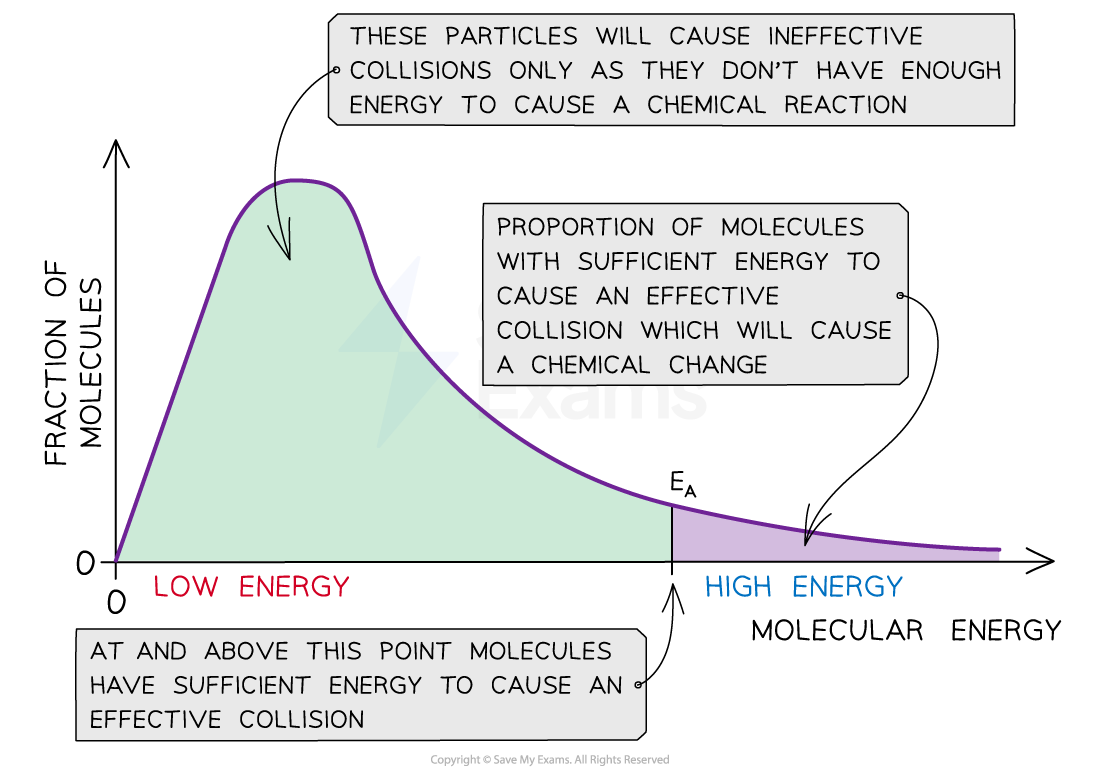
The Maxwell-Boltzmann curve shows the distribution of the energies of colliding particles and the activation energy.
When the temperature is increased, the distribution curve peak shifts to the right and flattens
This results from:
An increase in the average molecular energies of reacting molecules (peak shifts to the right)
An increase in the fraction of these molecules that have energies greater or equal to the activation energy (flattened curve)
However, the area under both curves, which represents the total number of particles, remains the same
Temperature Effect on Maxwell-Boltzmann Distribution Curve
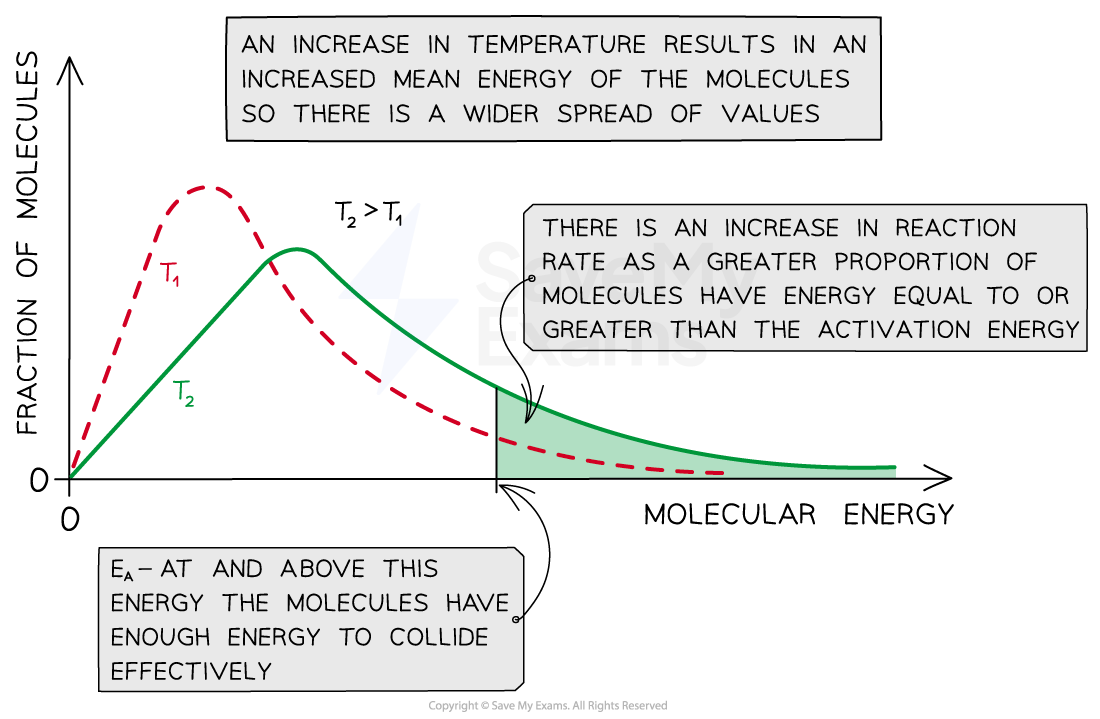
A graph showing the effect of temperature on the kinetic energy of reacting molecules. An increase in temperature increases the fraction of reactant molecules with the required activation energy

Unlock more, it's free!
Did this page help you?