Half-Life (Cambridge (CIE) O Level Physics): Revision Note
Exam code: 5054
Did this video help you?
Half-Life Basics
It is impossible to know when a particular unstable nucleus will decay
But the rate at which the activity of a sample decreases can be known
This is known as the half-life
Half-life is defined as:
The time taken for half the nuclei of that isotope in any sample to decay
In other words, the time it takes for the activity of a sample to fall to half its original level
Different isotopes have different half-lives and half-lives can vary from a fraction of a second to billions of years in length
Half-life can be determined from an activity–time graph
How to Determine Half-Life
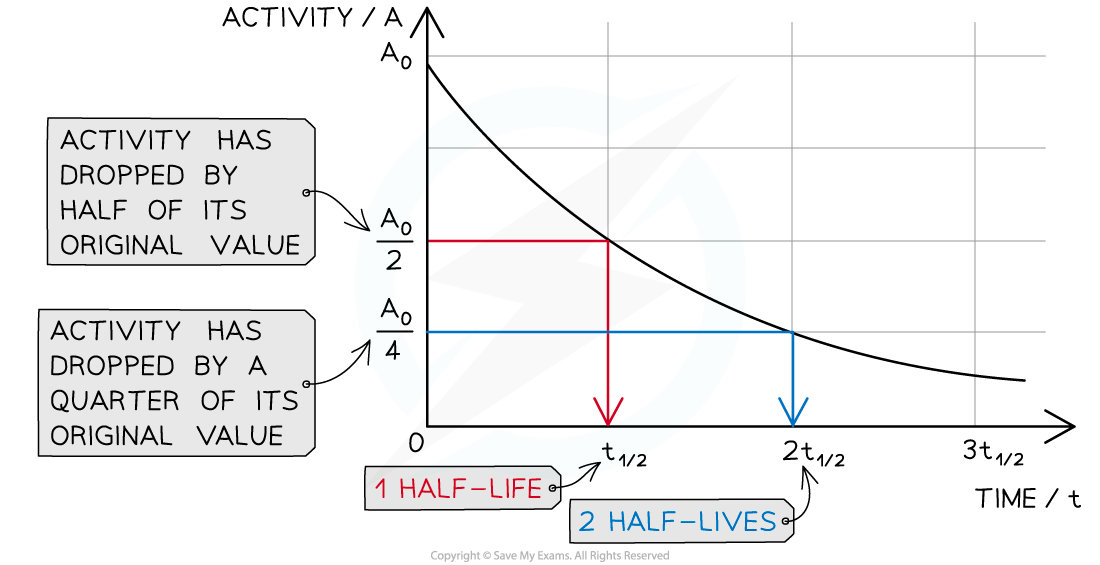
The graph shows how the activity of a radioactive sample changes over time. Each time the original activity halves, another half-life has passed
The time it takes for the activity of the sample to decrease from 100 % to 50 % is the half-life
It is the same length of time as it would take to decrease from 50 % activity to 25 % activity
The half-life is constant for a particular isotope
The table below shows that
As the number of half-lives increases by 1, the proportion of the isotope remaining halves
Relationship between the number of half-lives to the proportion of isotope remaining
Number of half-lives | Proportion of isotope remaining |
|---|---|
0 | 1 |
1 | |
2 | |
3 | |
4 |
Dating of Objects Using C-14
The isotope carbon-14 is commonly used in radioactive dating due to its abundance in living tissue
Carbon-14 forms when a high-energy cosmic ray knocks a neutron out of a nucleus, which then collides with a nitrogen nucleus in the air
This process can be represented by the equation:
Plants take in carbon dioxide from the atmosphere for photosynthesis, including the radioactive isotope carbon-14
Animals and humans take in carbon-14 by eating the plants
Therefore, all living organisms absorb carbon-14, but after they die they do not absorb any more
The proportion of carbon-14 is constant in living organisms as carbon is constantly being replaced during the period they are alive
When an organism dies, the activity of carbon-14 in the organic matter starts to fall, with a half-life of around 5730 years
Samples of living material can be tested by measuring the current amount of carbon-14 it contains and comparing this to the initial amount (which is based on the current ratio of carbon-14 to carbon-12)
The proportion of carbon-14 a sample contains can therefore provide an approximate age for the sample
Reliability of Carbon Dating
Carbon dating is a highly reliable ageing method for samples ranging from around 1000 to 40 000 years old
Therefore, for very young, or very old samples, carbon dating is not the most reliable method to use
This can be explained by looking at the decay curve of carbon-14:
Decay Curve for Carbon-14
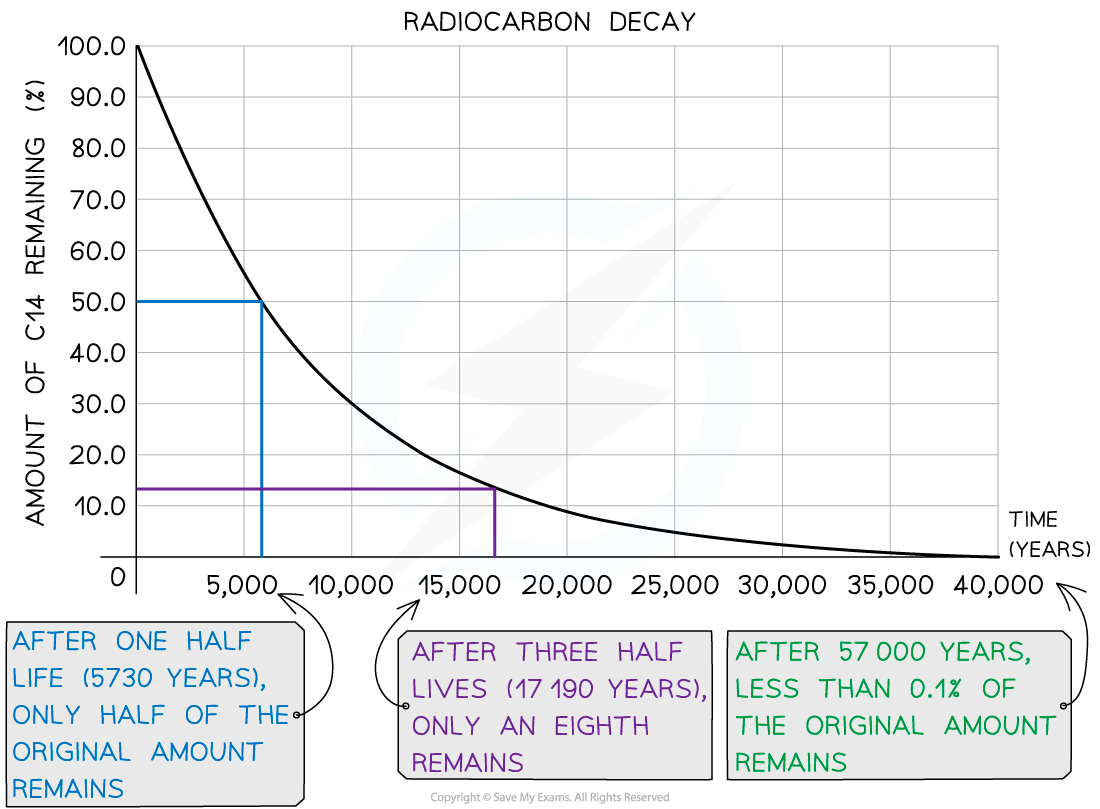
Carbon-14 decay curve used for carbon dating
If the sample is less than 1000 years old:
The activity of the sample will be too high
So, it is difficult to accurately measure the small change in activity
Therefore, the ratio of carbon-14 to carbon-12 will be too high to determine an accurate age
If the sample is more than 40 000 years old:
The activity will be too small and have a count rate similar to that of background radiation
So, there will be very few carbon-14 atoms remaining, hence very few decays will occur
Therefore, the ratio of carbon-14 to carbon-12 will be too small to determine an accurate age
Carbon dating uses the currently known ratio of carbon-14 to carbon-12, however, scientists cannot know the level of carbon-14 in the biosphere thousands of years ago
Therefore, this makes it difficult to age samples which are very old

Unlock more, it's free!
Did this page help you?