The Nucleus (Cambridge (CIE) O Level Physics): Revision Note
Exam code: 5054
Composition of the Nucleus
A nucleus is made up of positively charged protons and neutrally charged neutrons
The Protons, Neutrons and Electrons in an Atom

Protons and neutrons are found in the nucleus of an atom
Protons have a positive charge, whilst neutrons have no charge
This is why the nucleus is positive overall
Examiner Tips and Tricks
Be careful with your terminology:
Atom = nucleus (proton and neutron) and electrons
Nucleus = protons and neutrons at the centre of the atom
Atoms & Ions
An ion is an electrically charged atom or group of atoms formed by the loss or gain of electrons
An atom will lose or gain electrons to become more stable
A stable atom is normally electrically neutral
This means it has the same number of protons (positive charge) and electrons (negative charge)
Positive ions are therefore formed when atoms lose electrons
There will be more protons than electrons
Negative ions are therefore formed when atoms gain electrons
There will be more electrons than protons
Neutral, Positively and Negatively Charged Atoms

The difference between positive and negative ions depends on the number of elections
Examiner Tips and Tricks
You may hear the term 'net charge'. This just means the 'overall' charge of the atom. If an atom has 5 protons, 5 neutrons and 6 electrons, it has a net negative charge because it's a negative ion (more electrons than protons).
Remember which way around the charges are by proton being positive.
Did this video help you?
Describing the Nucleus
Proton Number, Z
The number of protons in an atom is called its proton number (it can also be called the atomic number)
Elements in the periodic table are ordered by their atomic number
Therefore, the number of protons determines which element an atom is
The atomic number of a particular element is always the same
For example:
Hydrogen has an atomic number of 1. It always has just one proton
Sodium has an atomic number of 11. It has 11 protons
Uranium has an atomic number of 92. It has 92 protons
The atomic number is also equal to the number of electrons in an atom
This is because atoms have the same number of electrons and protons in order to have no overall charge
Nucleon Number, A
The total number of particles in the nucleus of an atom is called its nucleon number (or mass number)
The mass number is the number of protons and neutrons in the atom
Calculating the Number of Neutrons
The number of neutrons can be found by subtracting the atomic number from the mass number
Number of Neutrons = Nucleon Number - Proton Number
For example, if a sodium atom has a mass number of 23 and an atomic number of 11, then the number of neutrons would be 23 – 11 = 12
Examiner Tips and Tricks
You may have noticed that the number of electrons is not part of the mass number. This is because electrons have a tiny mass compared to neutrons and protons. We say their mass is negligible when compared to the particles in the nucleus.
Nuclide Notation
A nuclide is a group of atoms containing the same number of protons and neutrons
For example, 5 atoms of oxygen are all the same nuclide but are 5 separate atoms
Atomic symbols are written in a specific notation called nuclide or ZXA notation
Nuclide Notation
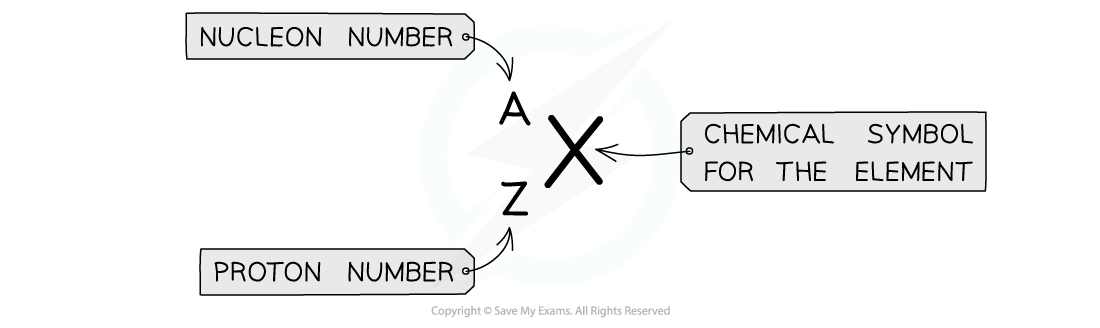
Atomic symbols in AZX Notation describe the constituents of nuclei
The top number A represents the nucleon number or the mass number
Nucleon number (A) = total number of protons and neutrons in the nucleus
The lower number Z represents the proton or atomic number
Proton number (Z) = total number of protons in the nucleus
Note: In Chemistry, the nucleon number is referred to as the mass number and the proton number as the atomic number.
The Atomic Symbol of Lithium
An example of an atomic symbol is:

Atomic symbols, like the one above, describe the constituents of nuclei
Worked Example
The element symbol for gold is Au. How many protons, neutrons and electrons are in the gold atom?

| Protons | Neutrons | Electrons |
|---|---|---|---|
A | 79 | 79 | 79 |
B | 197 | 79 | 118 |
C | 118 | 118 | 79 |
D | 79 | 118 | 79 |
Answer: D
Step 1: Determine the atomic and mass number
The gold atom has an atomic number of 79 (lower number) and a mass number of 197 (top number)
Step 2: Determine the number of protons
The atomic number is equal to the number of protons
The atom has 79 protons
Step 3: Calculate the number of neutrons
The mass number is equal to the number of protons and neutrons
The number of neutrons is equal to the mass number minus the atomic number
197 - 79 = 118
The atom has 118 neutrons
Step 4: Determine the number of electrons
The atom has 79 electrons
Did this video help you?
Isotopes
Although the number of protons in a particular element is always the same, the number of neutrons can be different
Isotopes are atoms of the same element that have an equal number of protons but a different number of neutrons
This means that each element can have more than one isotope
Isotopes tend to be more unstable due to their imbalance of protons and neutrons
This means they're more likely to decay
In the diagram below are three isotopes of Hydrogen:
The Three Isotopes of Hydrogen
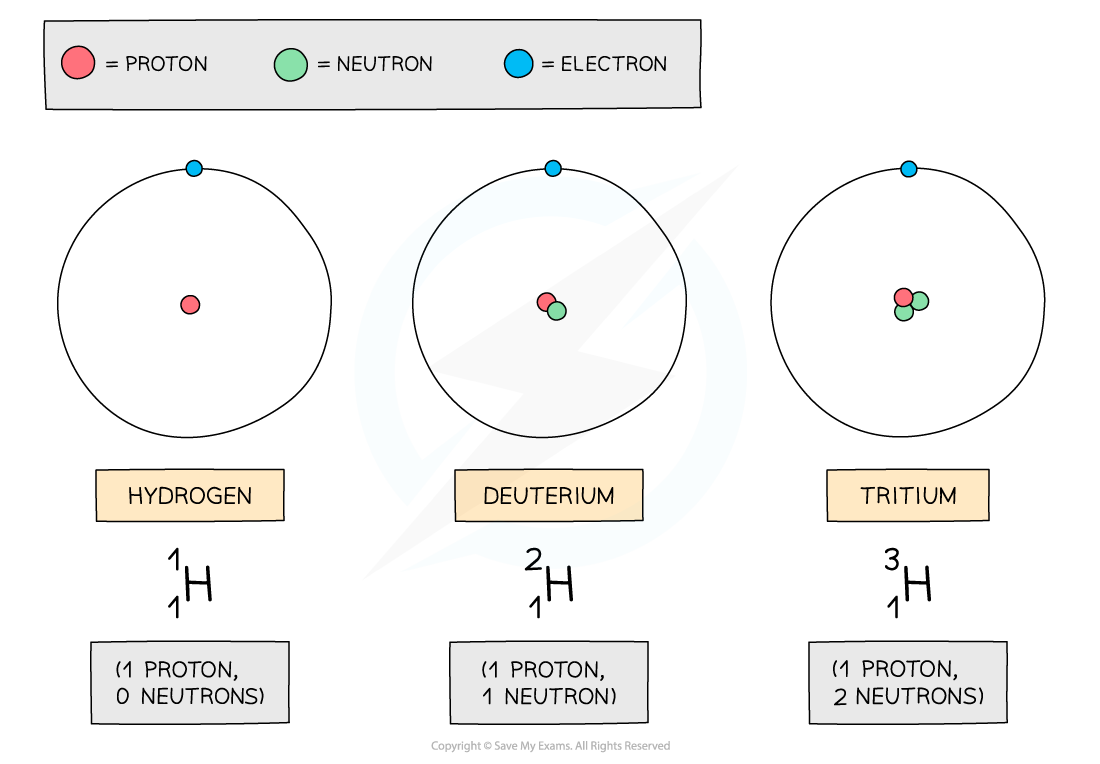
Hydrogen has three isotopes, each with a different number of neutrons
Isotopes occur naturally, but some are more rare than others
For example, about 2 in every 10,000 Hydrogen atoms is Deuterium
Tritium is even more rare (about 1 in every billion billion hydrogen atoms)
Worked Example
Which of the following elements are isotopes of each other?
A |
|
B |
|
C |
|
D |
|
Answer: B
In nuclide notion, the top number is the nucleon number (number of protons and neutrons) and the bottom number is the proton number (number of protons)
Isotopes are two of the same elements
This eliminates option D since one is oxygen (O) and the other nitrogen (N)
Which have the same number of protons
This eliminates options C and A
Their proton numbers are different for the same element
But a different number of neutrons
Therefore, the correct answer is B

Unlock more, it's free!
Was this revision note helpful?
