Investigating Specific Heat Capacity (Cambridge (CIE) O Level Physics) : Revision Note
Investigating Specific Heat Capacity
Aims of the Experiment
The aim of the experiment is to determine the specific heat capacity of a substance, by linking the decrease of one energy store (or work done) to the increase in temperature and subsequent increase in thermal energy stored
Variables
Independent variable = Time, t
Dependent variable = Temperature, θ
Control variables:
Material of the block
Current supplied, I
Potential difference supplied, V
Equipment List
EquipmentPurposeSubstance to calculate the specific heat capacity1kg block of metal (e.g. aluminium), or, a beaker containing a known mass of waterThermometerTo measure the temperature rise of the substanceImmersion heaterTo heat the substancePower supplyTo supply power to the heaterStopwatchTo measure the time taken for the substance to heat up by a certain temperatureVoltmeterTo determine the potential difference through the heaterAmmeterTo determine the current from the power supply to the heater
Equipment | Purpose |
|---|---|
1kg block of metal (e.g. aluminium), or, a beaker containing a known mass of water | Substance to calculate the specific heat capacity |
Thermometer | To measure the temperature rise of the substance |
Immersion heater | To heat the substance |
Power supply | To supply power to the heater |
Stopwatch | To measure the time taken for the substance to heat up by a certain temperature |
Voltmeter | To determine the potential difference through the heater |
Ammeter | To determine the current from the power supply to the heater |
Resolution of measuring equipment:
Thermometer = 1 °C
Stopwatch = 0.01 s
Voltmeter = 0.1 V
Ammeter = 0.01 A
Method
Apparatus to Measure Specific Heat Capacity
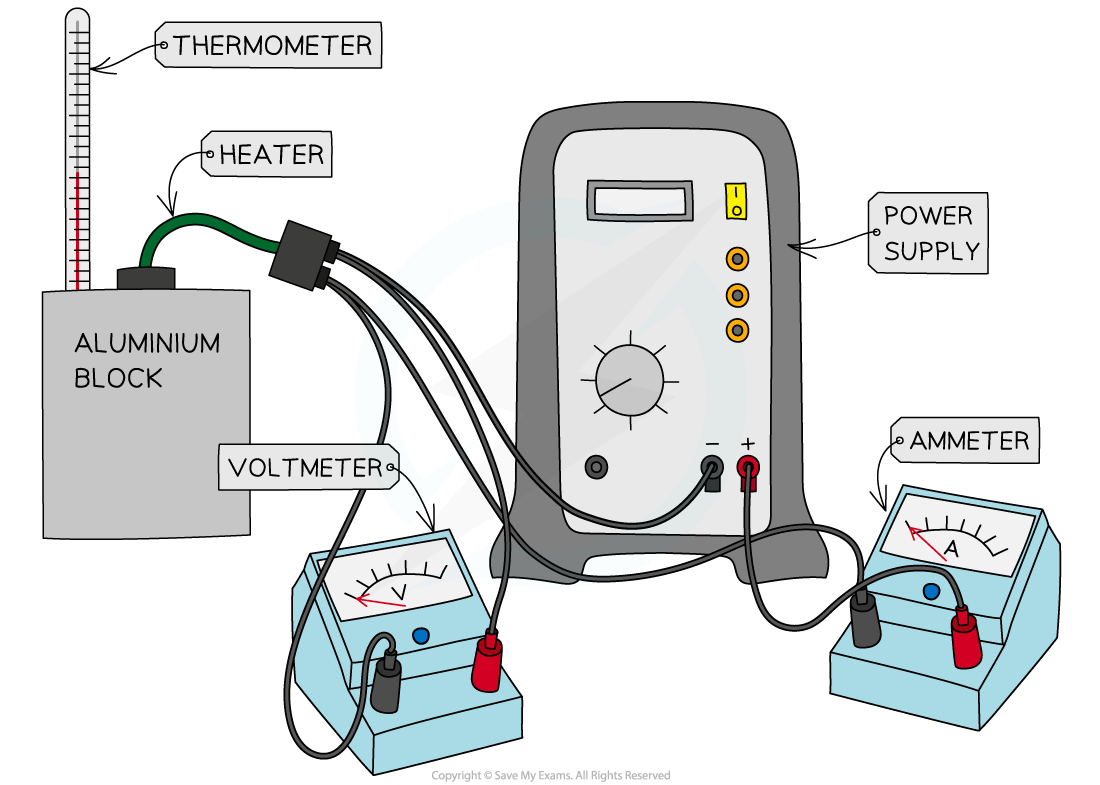
Apparatus to investigate the specific heat capacity of the aluminium block
Start by assembling the apparatus, placing the heater into the top of the block
Measure the initial temperature of the aluminium block from the thermometer
Turn on the power supply and start the stopwatch
Whilst the power supply is on, the heater will heat up the block. Take several periodic measurements, eg. every 1 minute of the voltage and current from the voltmeter and ammeter respectively, calculating an average for each at the end of the experiment up to 10 minutes
Switch off the power supply, stop the stopwatch and leave the apparatus for about a minute. The temperature will still rise before it cools
Monitor the thermometer and record the final temperature reached for the block
An example table of results might look like this:
Example Results Table

Analysis of Results
The thermal energy supplied to the block can be calculated using the equation:
E = IVt
Where:
E = thermal energy, in joules (J)
I = current, in amperes (A)
V = potential difference, in volts (V)
t = time, in seconds (s)
The change in thermal energy is defined by the equation:
ΔE = mcΔθ
Where:
ΔE = change in thermal energy, in joules (J)
m = mass, in kilograms (kg)
c = specific heat capacity, in joules per kilogram per degree Celsius (J/kg °C)
Δθ = change in temperature, in degrees Celsius (°C)
Rearranging for the specific heat capacity, c:
To calculate Δθ:
Δθ = final temperature – initial temperature
To calculate ΔE:
ΔE = IVtf – IVti
Where:
I = average current, in amperes (A)
V = average potential difference (V)
tf = final time, in seconds (s)
ti = initial time, in seconds (s)
These values are then substituted into the specific heat capacity equation to calculate the specific heat capacity of the aluminium block
Evaluating the Experiment
Systematic Errors:
Make sure the voltmeter and ammeter are initially set to zero, to avoid zero error
Random Errors:
Not all the heat energy supplied from the heater will be transferred to the block, some will go into the surroundings or heat up the thermometer
This means the measured value of the specific heat capacity is likely to be higher than what it actually is
To reduce this effect, make sure the block is fully insulated
A joulemeter could be used to calculate energy directly
This would eliminate errors from the voltmeter, ammeter and the stopwatch
Make sure the temperature value is read at eye level from the thermometer, to avoid parallax error
The experiment can also be repeated with a beaker of water of equal mass, the water should heat up slower than the aluminium block
Safety Considerations
Make sure never to touch the heater whilst it is on, otherwise, it could burn skin or set something on fire
Run any burns immediately under cold running water for at least 5 minutes
Allow time for all the equipment, including the heater, wire and block to cool before packing away the equipment
Keep water away from all electrical equipment
Wear eye protection if using a beaker of hot water

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
