Temperature & Pressure (Cambridge (CIE) O Level Physics): Revision Note
Exam code: 5054
Temperature & Energy of Particles
Molecules in a gas move around in constant random motion at high speeds
Random motion means that molecules
Travel in no specific direction
Undergo sudden changes in direction if they collide with either the walls of the container, or with other molecules
Random Motion of Gas Molecules

Gas molecules in a container move around randomly at high speeds
The motion of molecules in a gas depends on the temperature of the gas
More specifically, the temperature of a gas is related to the average kinetic energy of the molecules
The hotter the gas, the higher the average kinetic energy
The cooler the gas, the lower the average kinetic energy
Since the kinetic energy is related to speed, the speed of the molecules also changes with temperature (as long as the volume of the container is constant)
The hotter the gas, the faster the gas molecules move
The cooler the gas, the slower the gas molecules move
Absolute Zero
In 1848, the physicist Lord Kelvin recognised that there must be a temperature at which the particles in a gas must no longer be moving, or exerting pressure on their surroundings
This temperature is called absolute zero and is equal to −273 °C
Absolute zero is defined as:
The temperature at which the molecules in a substance have zero kinetic energy
This means for a system at absolute zero, it is not possible to remove any more energy from it
Even in space, the temperature is roughly 2.7°C above absolute zero
Where does absolute zero come from?

At absolute zero (−273°C) particles will have no net movement. It is therefore not possible to have a lower temperature than this
Motion of Particles in a Gas
A feature of gases is that they fill the space of any container that they occupy
As the gas particles move about randomly they collide with the walls of the container and exert a pressure
These collisions produce a net force at right angles to the wall of the container (or any surface)
This pressure is defined as the force per unit area
Where:
P = pressure exerted by the gas (Pa)
F = force exerted by the gas (N)
A = area the force acts over (m2)
This equation means that
The higher the gas pressure, the more frequently the particles collide with the container walls and the greater the force exerted per unit area
The lower the gas pressure, the less frequently the particles collide with the container walls and the smaller the force exerted per unit area
Pressure & Force in a Gas

Gas molecules bouncing off the walls of a container
The pressure of a gas also depends on the temperature of the gas
This is because particles move with more energy as their temperature increases
As the temperature of the gas decreases, the pressure on the container also decreases
Gas Molecules in a Container
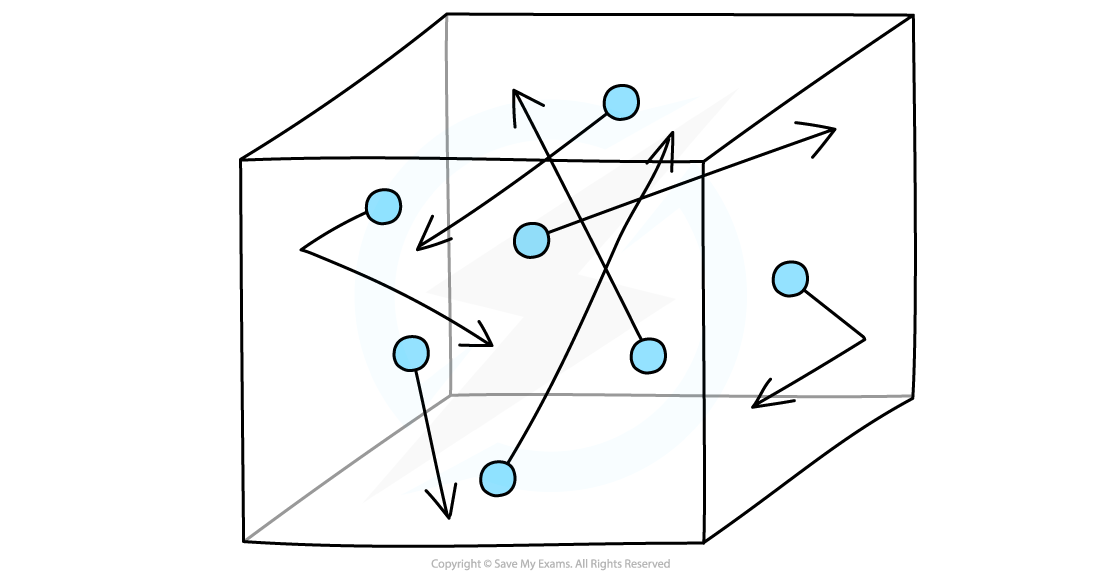
Gas molecules hit the sides of the container and exert a force, which creates pressure
Examiner Tips and Tricks
You can experience the force exerted by a gas yourself by closing your mouth and forcing air into your cheeks
The strain you feel on your cheeks is due to the increased pressure of the gas particles pushing at right angles to your cheeks

Unlock more, it's free!
Did this page help you?