Electrolysis of Aqueous Solutions (Cambridge (CIE) O Level Chemistry) : Revision Note
Did this video help you?
Electrolysis of Aqueous Solutions
Aqueous solutions will always have water present (H2O)
In the electrolysis of aqueous solutions, the water molecules dissociate producing H+ and OH– ions:
H2O ⇌ H+ + OH–
These ions are also involved in the process and their chemistry must be considered
We now have an electrolyte that contains ions from the compound plus ions from the water
Which ions get discharged and at which electrode depends on the relative reactivity of the elements involved
Concentrated and dilute solutions of the same compound give different products
For anions, the more concentrated ion will tend to get discharged over a more dilute ion
Positive Electrode (anode)
Negatively charged OH– ions and non-metal ions are attracted to the positive electrode
If halide ions (Cl-, Br-, I-) and OH- are present then the halide ion is discharged at the anode, loses electrons and forms a halogen (chlorine, bromine or iodine)
If no halide ions are present, then OH- is discharged at the anode, loses electrons and forms oxygen gas
In both cases the other negative ion remains in solution
The concentration of the solution also affects which ion is discharged:
If a concentrated halide solution is being electrolysed, the halogen forms at the anode
If a dilute halide solution is being electrolysed, oxygen is formed
For example:
For a concentrated solution of barium chloride, the Cl- ions are discharged more readily than the OH- ions, so chlorine gas is produced at the anode
If the solution is dilute however only the OH- ion is discharged and so oxygen would be formed
Negative Electrode (cathode)
Positively charged H+ and metal ions are attracted to the negative electrode but only one will gain electrons
Either hydrogen gas or metal will be produced
If the metal is above hydrogen in the reactivity series, then hydrogen will be produced and bubbling will be seen at the cathode
This is because the ions of the more reactive metal will remain in the solution, causing the ions of the least reactive metal to be discharged
Therefore, at the cathode, hydrogen gas will be produced unless the positive ions from the ionic compound are less reactive than hydrogen, in which case the metal is produced
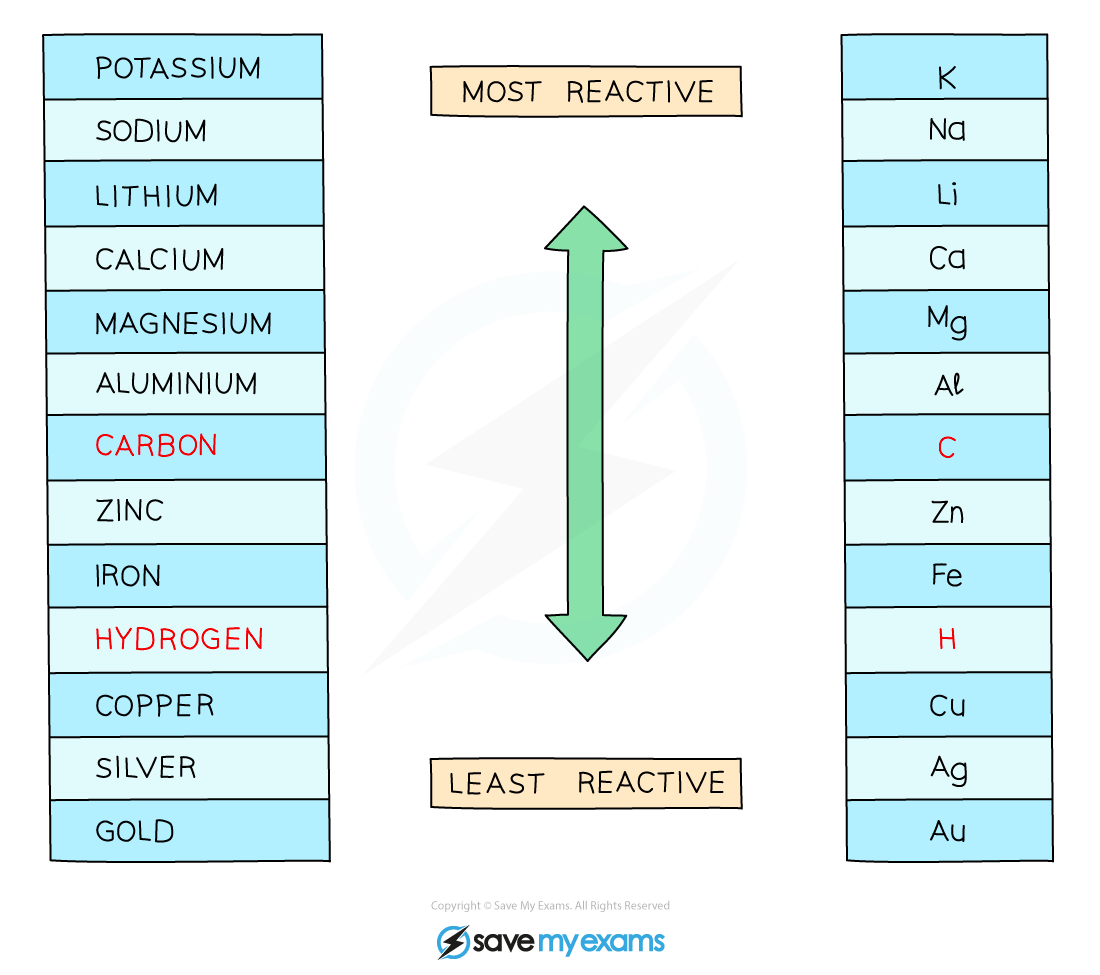
The reactivity series of metals including hydrogen and carbon
Products formed for Common Aqueous Solutions

Electrolysis of Aqueous Copper(II) Sulfate
Electrolysis of Aqueous Copper Sulfate
Aqueous copper sulfate contains the following ions:
Cu2+, SO42-, H+ and OH-
Using graphite electrodes:

Apparatus for the electrolysis of copper(II)sulfate using inert / passive graphite electrodes
Product at the Cathode:
Cu2+ and H+ will both be attracted to the cathode but the less reactive ion will be discharged
In this case, copper is less reactive than hydrogen
Copper ions are discharged at the cathode, gain electrons and are reduced to form copper metal
The half equation for the reaction at the electrode is:
Cu2+ + 2e- → Cu
Product at the Anode:
SO42- and OH- are both attracted to the anode
OH- ions lose electrons more readily than SO42-
OH- lose electrons and are oxidised to form oxygen gas
The half equation for the reaction at the anode is
4OH– ⟶ O2 + 2H2O + 4e–
Using copper electrodes:
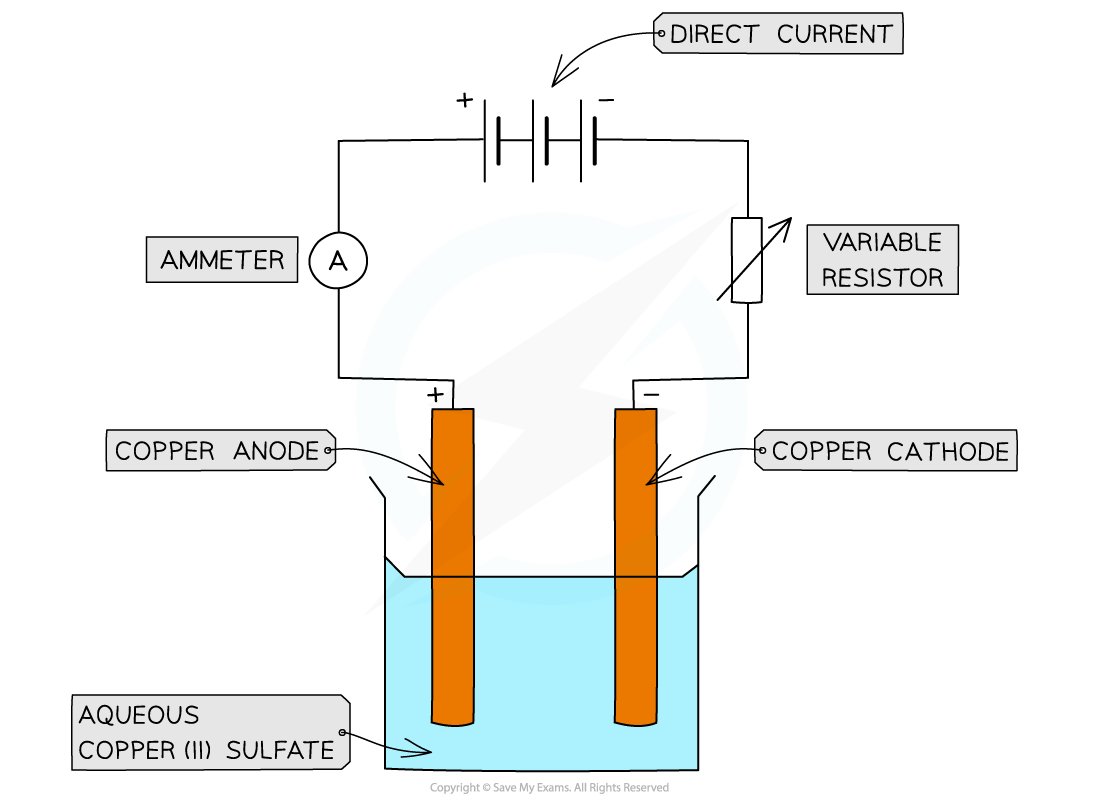
Apparatus for the electrolysis of copper(II)sulfate using active copper electrodes
Observations at the anode and cathode
The cathode increases in mass while the anode decreases
This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the cathode, forming copper atoms
The gain in mass by the negative electrode is the same as the loss in mass by the positive electrode
Therefore the copper deposited on the negative electrode must be the same copper ions that are lost from the positive electrode
That implies that the concentration of the Cu2+ ions in the solution remains constant

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
