Isotopes (Cambridge (CIE) O Level Chemistry): Revision Note
Exam code: 5070
Defining Isotopes
Isotopes are different atoms of the same element that contain the same number of protons but a different number of neutrons
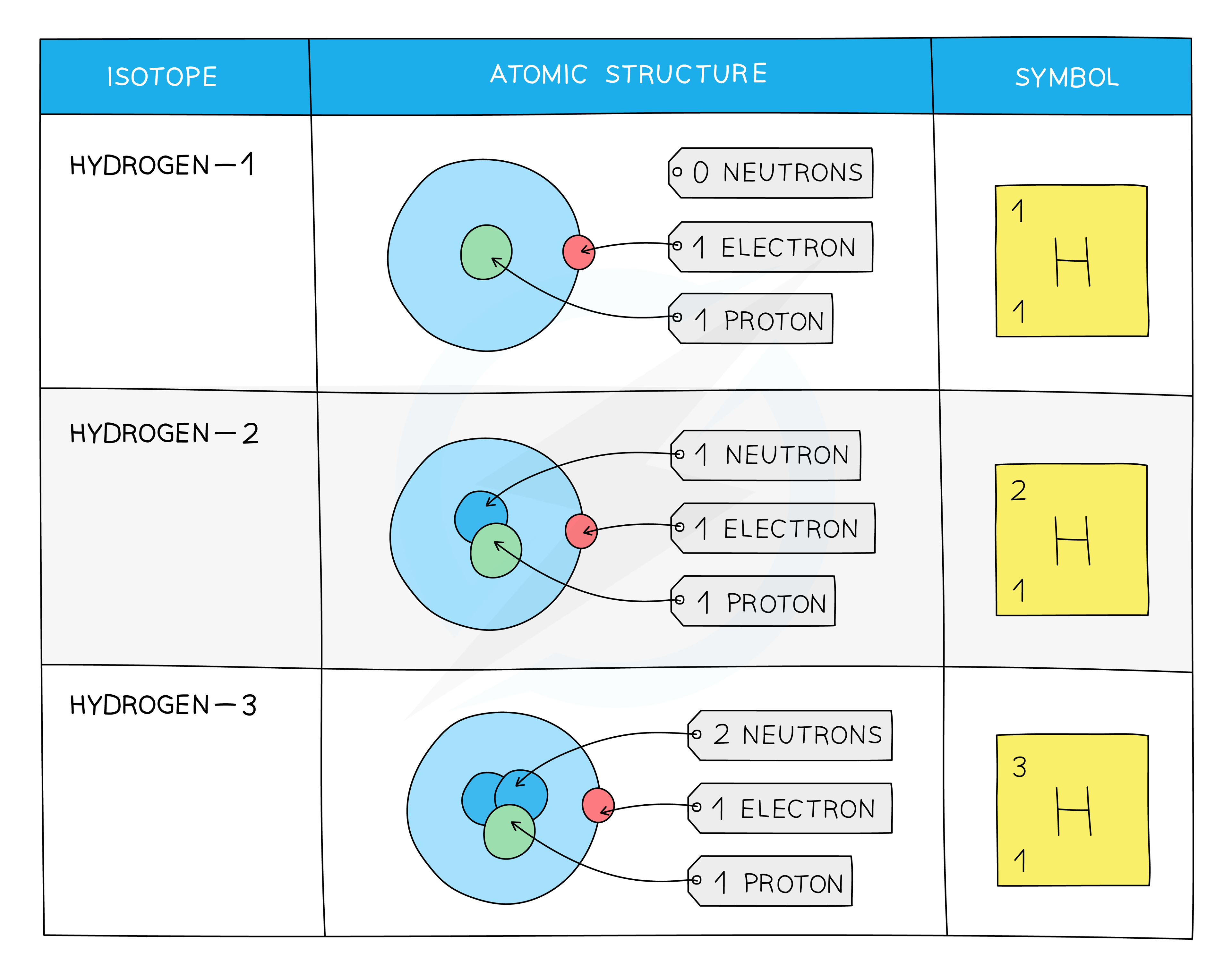
The symbol for an isotope is the chemical symbol (or word) followed by a dash and then the mass number
So C-14 ( or carbon-14) is the isotope of carbon which contains 6 protons, 6 electrons and 14 - 6 = 8 neutrons
It can also be written as 14C or
The Atomic Structure and Symbols of the Three Isotopes of Hydrogen
Why Isotopes Share Properties
Isotopes of the same element display the same chemical characteristics
This is because they have the same number of electrons in their outer shells and, therefore, the same electronic configuration and this is what determines an atom's chemistry
The difference between isotopes is the number of neutrons which are neutral particles within the nucleus and add mass only
The difference in mass affects the physical properties, such as density, boiling point and melting point
Isotopes are identical in appearance, so a sample of C-14 would look no different from C-12
Water made from deuterium oxide is known as 'heavy' water, and has a relative formula of mass 20, compared to 18 for water, so it is 20% heavier, but it would look, taste and feel just like normal water
However, it wouldn't be a good idea to drink it because it is toxic as it interferes with biochemical reactions in your cells!
Calculating Relative Atomic Mass
Relative Atomic Mass
The symbol for the relative atomic mass is Ar
The relative atomic mass for each element can be found in the Periodic Table along with the atomic number
The atomic number is shown above the atomic symbol and the relative atomic mass is shown below the atomic symbol
Atoms are too small to accurately weigh but scientists needed a way to compare the masses of atoms
The carbon-12 is used as the standard atom and has a fixed mass of 12 units
It is against this atom which the masses of all other atoms are compared
Relative atomic mass (Ar) can therefore be defined as:
the average mass of the isotopes of an element compared to 1/12th of the mass of an atom of 12C
The relative atomic mass of carbon is 12
The relative atomic mass of magnesium is 24 which means that magnesium is twice as heavy as carbon
The relative atomic mass of hydrogen is 1 which means it has one-twelfth the mass of one carbon-12 atom
The relative atomic mass of an element can be calculated from the mass number and relative abundances of all the isotopes of a particular element using the following equation:
The top line of the equation can be extended to include the number of different isotopes of a particular element present.
Example
The table shows information about the isotopes in a sample of rubidium

Are mass number and relative atomic mass the same thing?
On the Periodic Table provided in your exam you will see that lithium has a relative atomic mass of 7
Although it seems that this is the same as the mass number, they are not the same thing because the relative atomic mass is a rounded number
Relative atomic mass takes into account the existence of isotopes when calculating the mass
Relative atomic mass is an average mass of all the isotopes of that element
For simplicity relative atomic masses are often shown to the nearest whole number

The relative atomic mass of lithium to two decimal places is 6.94 when rounded to the nearest whole number, the RAM is 7, which is the same as the mass number shown on this isotope of lithium

Unlock more, it's free!
Was this revision note helpful?