Isotopes & Mass Spectra (Edexcel International AS Chemistry) : Revision Note
The Mass Spectrometer
The mass spectrometer is an instrument used to determine the relative isotopic mass and the relative abundance of each isotope
Relative isotopic mass is the mass of an individual isotope relative to the mass of carbon-12
Apart from finding the masses of isotopes, mass spectrometry is a vey useful tool for detecting illegal drugs, forensic science, space exploration and carbon-14 dating

The components of a mass spectrometer
How the mass spectrometer works
A sample is injected into the spectrometer where it is heated and vaporised
The atoms or molecules are then bombarded by high energy electrons created by an electron gun
The high energy electrons collide with the sample material and remove electrons creating positive ions
The ions are then accelerated by attraction towards negatively charged plates
Many ions strike the plate and are discharged, but a few will pass through a gap in the plates into a curved section of the spectrometer known as the flight tube
The flight tube is encased in electromagnets which create a strong electromagnetic field that deflects the path of the ions in a curve
The ions pass onto a charged detector plate where every strike is recorded as a small current which is then amplified
By varying the electric and magnetic fields all the charged particles can be deflected to the detector which gives information about the mass/charge ratio and abundance of each ion
Isotopes & Mass Spectra
Isotopes are different atoms of the same element that contain the same number of protons and electrons but a different number of neutrons
These are atoms of the same elements but with different mass numbers
Therefore, the mass of an element is given as relative atomic mass (Ar) by using the average mass of the isotopes
The relative atomic mass of an element can be calculated by using the relative abundance values
Ar =
The relative abundance of an isotope is either given or can be read off the mass spectrum
Worked Example
Calculating the relative atomic mass of oxygen
A sample of oxygen contains the following isotopes:
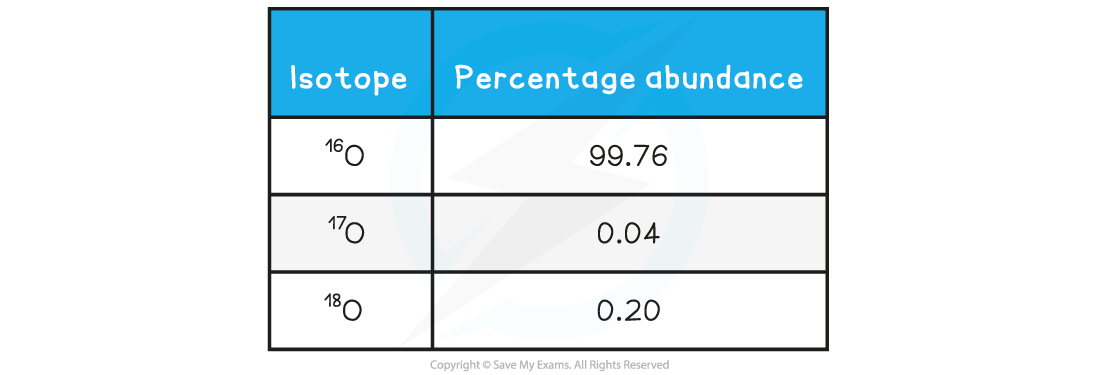
What is the relative atomic mass, Ar, of oxygen in this sample, to 2dp?
Answer
Ar =
Ar = 16.0044
Ar = 16.00
Worked Example
Calculating the relative atomic mass of boron
Calculate the relative atomic mass of boron using its mass spectrum, to 1dp:

Answer
Ar =
Examiner Tips and Tricks
You can be expected to work with tables or graphs of data to calculate relative atomic mass
You can also be expected to do these calculations backwards to determine the abundance of one isotope given sufficient information
Mass spectra of molecules
When a compound is analysed in a mass spectrometer, vaporised molecules are bombarded with a beam of high-speed electrons
These knock off an electron from some of the molecules, creating molecular ions:

The relative abundances of the detected ions form a mass spectrum: a kind of molecular fingerprint that can be identified by computer using a spectral database
The peak with the highest m/z value is the molecular ion (M+) peak which gives information about the molecular mass of the compound
This value of m/z is equal to the relative molecular mass of the compound
The M+1 peak
The [M+1] peak is a smaller peak which is due to the natural abundance of the isotope carbon-13
The height of the [M+1] peak for a particular ion depends on how many carbon atoms are present in that molecule; The more carbon atoms, the larger the [M+1] peak is
For example, the height of the [M+1] peak for an hexane (containing six carbon atoms) ion will be greater than the height of the [M+1] peak of an ethane (containing two carbon atoms) ion
Worked Example
Determine whether the following mass spectrum belongs to propanal or butanal

Answer:
The mass spectrum corresponds to propanal as the molecular ion peak is at m/z = 58
Propanal arises from the CH3CH2CHO+ ion which has a molecular mass of 58
Butanal arises from the CH3CH2CH2CHO+ ion which has a molecular mass of 72
Examiner Tips and Tricks
A mass spectrum can give lots of information about fragments of the overall compound being analysed
Mass Spectra - Predictions
You can also predict how a mass spectrum might appear for a given compound, e.g. ethanol, CH3CH2OH
The methyl, CH3+, fragment has a mass of 15.0
The ethyl, CH3CH2+, fragment has a mass of 29.0
The base ion, CH2OH+, fragment has a mass of 31.0
The whole molecule has a mass of 46.0
Predicting mass spectra becomes more complex with the inclusion of halogen isotopes such as chlorine and bromine
Chlorine
Chlorine exists as two isotopes, 35Cl and 37Cl
A compound containing one chlorine atom will therefore have two molecular ion peaks due to the two different isotopes it can contain
35Cl = M+ peak
37Cl = [M+2] peak
The ratio of the peak heights is 3:1 (as the relative abundance of 35Cl is 3x greater than that of 37Cl)
A diatomic chlorine molecule or a compound containing two chlorine atoms will have three molecular ion peaks due to the different combinations of chlorine isotopes they can contain
35Cl + 35Cl = M+ peak
35Cl + 37Cl = [M+2] peak
There is an alternative of 37Cl + 35Cl doubling the [M+2] peak
37Cl + 37Cl = [M+4] peak
The ratio of the peak heights is 9:6:1
This ratio can be deduced by using the probability of each chlorine atom being 35Cl or 37Cl
35Cl + 35Cl =
35Cl + 37Cl =
but this doubles for the 37Cl + 35Cl option, therefore,
37Cl + 37Cl =
The presence of bromine or chlorine atoms in a compound gives rise to a [M+2] and possibly [M+4] peak

Mass spectrum of compounds containing one chlorine atom (1) and two chlorine atoms (2)
Bromine
Bromine too exists as two isotopes, 79Br and 81Br
A compound containing one bromine atom will have two molecular ion peaks
79Br = M+ peak
81Br = [M+2] peak
The ratio of the peak heights is 1:1 (they are of similar heights as their relative abundance is the same!)
A diatomic molecule of bromine or a compound containing two bromine atoms will have three molecular ion peaks
79Br + 79Br= M+ peak
79Br+ 81Br = [M+2] peak
81Br + 81Br= [M+4] peak
The ratio of the peak heights is 1:2:1

Mass spectrum of compounds containing one bromine atom

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?
