Alpha, Beta & Gamma Radiation (Edexcel International A Level (IAL) Physics) : Revision Note
Alpha, Beta & Gamma Particles
Some elements have nuclei that are unstable
This tends to be when the number of nucleons does not balance
In order to become more stable, they emit particles and/or electromagnetic radiation
These nuclei are said to be radioactive
There are three different types of radioactive emission: Alpha, Beta and Gamma
Alpha Particles
Alpha (α) particles are high energy particles made up of 2 protons and 2 neutrons (the same as a helium nucleus)
They are usually emitted from nuclei that are too large

Beta Particles
Beta (β−) particles are high energy electrons emitted from the nucleus
β− particles are emitted by nuclei that have too many neutrons

Beta is a moderately ionising type of radiation
This is due to it having a charge of +1e
This means it is able to do some slight damage to cells (less than alpha but more than gamma)
Beta is a moderately penetrating type of radiation
Beta particles have a range of around 20 cm - 3 m in air, depending on their energy
Beta can be stopped by a few millimetres of aluminium foil
Gamma Rays
Gamma (γ) rays are high energy electromagnetic waves
They are emitted by nuclei that need to lose some energy

If these particles hit other atoms, they can knock out electrons, ionising the atom
This can cause chemical changes in materials and can damage or kill living cells
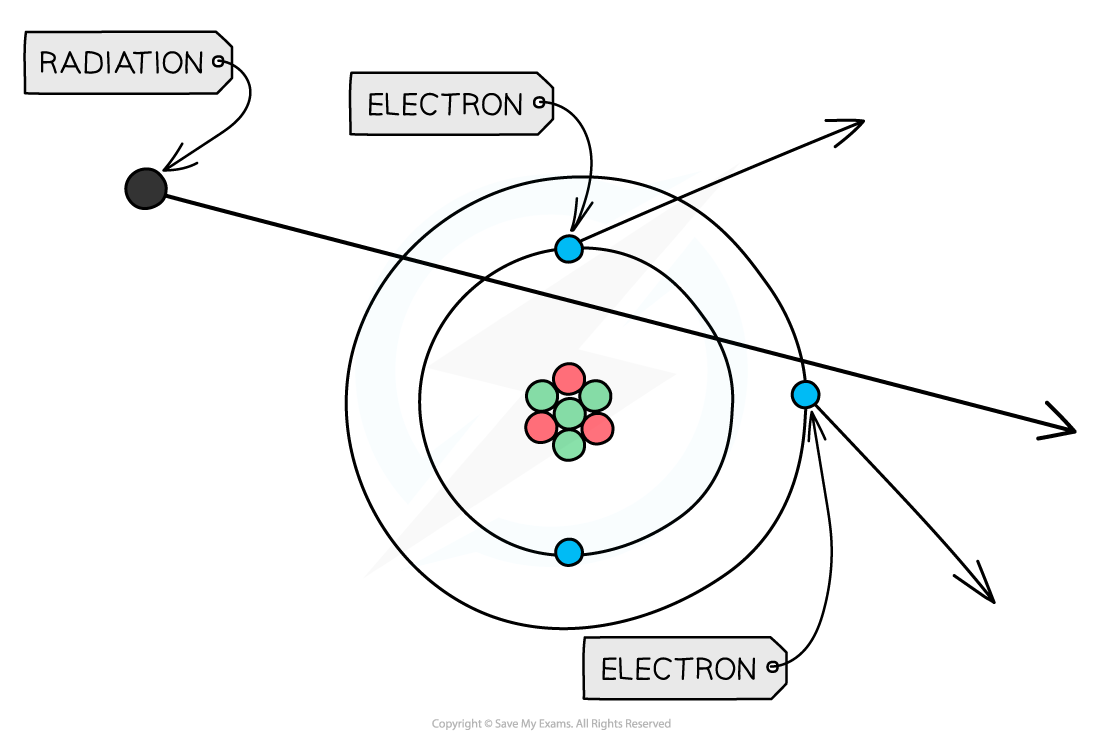
When radiation passes close to atoms, it can knock out electrons, ionising the atom
The properties of the different types of radiation are summarised in the table below

u is the atomic mass unit (see “Atomic Mass Unit (u)”)
e is the charge of the electron: 1.60 × 10-19 C
c is the speed of light: 3 × 108 m s-1
Worked Example

Answer: D

Examiner Tips and Tricks
It is important to be familiar the properties of each type of radiation and their symbols.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
