Nucleon & Proton Number (Edexcel International A Level (IAL) Physics) : Revision Note
Nucleon & Proton Number
Atomic symbols are written in a specific notation as shown below:
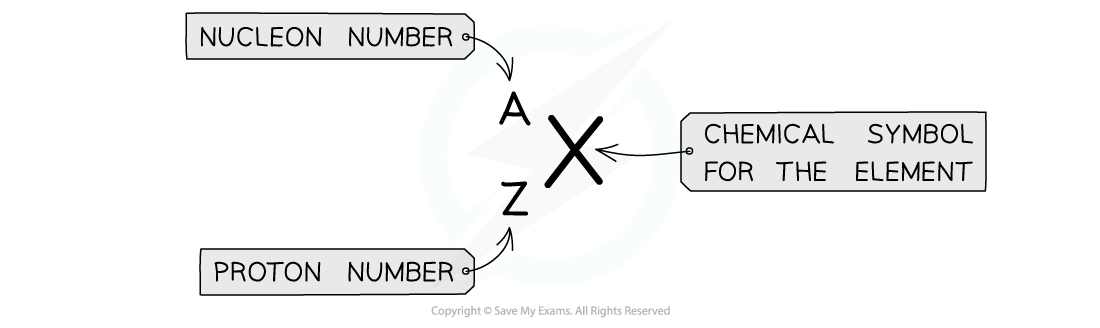
Atomic symbols show the proton number and nucleon number
The top number A represents the nucleon number or the mass number
Nucleon number (A) = total number of protons and neutrons in the nucleus
The lower number Z represents the proton or atomic number
Proton number (Z) = total number of protons in the nucleus
Note: In Chemistry, the nucleon number is referred to as the mass number and the proton number as the atomic number. The periodic table is ordered by atomic number
Isotopes
Although all atoms of the same element always have the same number of protons (and hence electrons), the number of neutrons can vary
An isotope is an atom (of the same element) that has an equal number of protons but a different number of neutrons
For example, hydrogen has two isotopes: deuterium and tritium. Both isotopes have a proton number of 1
Deuterium has one neutron, so its nucleon number is 2
Tritium has two neutrons, so its nucleon number is 3
Worked Example


Examiner Tips and Tricks
Remember which number in the chemical notation is the nucleon number and proton number is vital for many topics involving particle decays e.g. radioactivity. The most common mistake is thinking the nucleon number is the number of neutrons, the number of neutrons is calculated by:
number of neutrons = nucleon number – proton number
In all neutral (uncharged) atoms, the number of protons = number of electrons.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
