Atomic Line Spectra (Edexcel International A Level (IAL) Physics) : Revision Note
Atomic Line Spectra
An emission line spectrum is produced when:
An excited electron in an atom moves from a higher to a lower energy level and emits a photon with an energy corresponding to the difference between these energy levels
Each element produces a unique emission line spectrum due to its unique set of energy levels
Hot gases produce emission line spectra, such as stars
When the atoms of a gas are excited, electrons gain energy and move to higher energy levels

When an electron moves from a higher energy level to a lower energy level, a photon is released
Electrons cannot stay in a continuous state of excitation, so they will move back to lower energy levels through de-excitation
During de-excitation, energy must be conserved, so transitions result in an emission of photons with discrete frequencies (or wavelengths) specific to that element
Since there are many possible electron transitions for each atom, there are many different radiated wavelengths
This creates a line spectrum consisting of a series of bright lines against a dark background
An emission line spectrum acts as a fingerprint of the element

An example of the emission line spectrum of hydrogen
Each line of the emission spectrum corresponds to a different energy level transition within the atom
Electrons can transition between energy levels absorbing or emitting a discrete amount of energy
An excited electron can transition down to the next energy level or move to a further level closer to the ground state
For example, if an atom has six energy levels:
At low temperatures, most electrons will occupy the ground state n = 1
At high temperatures, electrons may be excited to the most excited state n = 6

Energy and frequency of a photon are directly proportional
The energy of a photon is given by the equation:
E = hf
Using energy can be written as:
E =
Where:
E = energy of the photon (J)
h = Planck's constant(J s)
c = speed of light (m s-1)
f = frequency (Hz)
λ = wavelength (m)
The energy required to move from one energy level to another is given by the difference of energy between the two energy levels:
ΔE = E1 – E2
Where:
E1 = energy associated with the level that the electron has left (eV)
E2 = energy associated with the level that the electron moves to (eV)
The difference of energy corresponds to the energy of the absorbed (or emitted) photon:
ΔE = E1 – E2 = hf =
For each transition, a photon will be emitted with a specific wavelength
In the case of hydrogen, all wavelengths are in the visible range:
From n = 6 to n = 2 - violet
From n = 5 to n = 2 - blue
From n = 4 to n = 2 - light blue
From n = 3 to n = 2 - red

If the emitted photons are in the visible range, wavelengths can be represented as lines of the respective colour against a black background
Emitted photons can have a range of wavelengths spanning the whole electromagnetic spectrum
The wavelength is inversely proportional to the energy level transition associated with the emitted photon
For example, the transitions for hydrogen will be as follows:
To n = 1 (ground level) – ultraviolet, highest energy, high frequency, short wavelength
To n = 2 – visible light, violet is the highest energy, red is the lowest energy
To n = 3 – infrared light, lowest energy, low frequency, longest wavelength
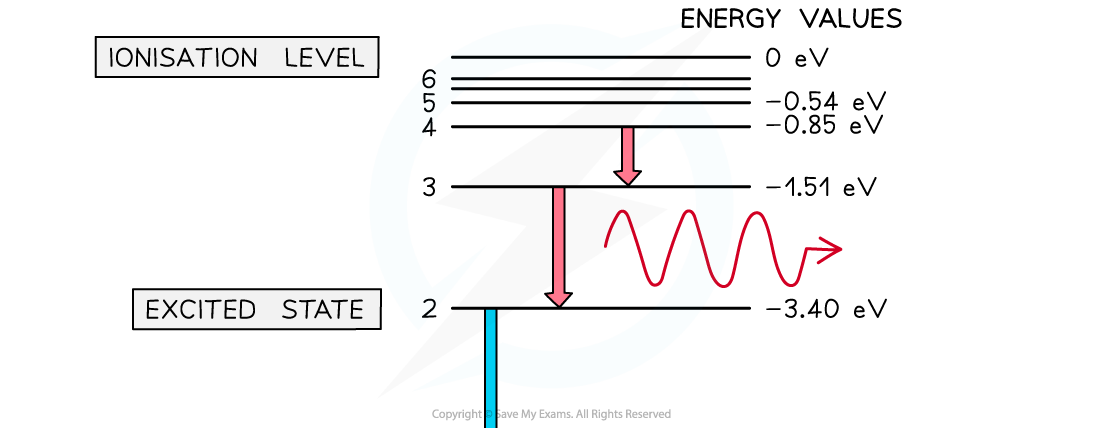

The larger the energy transition, the longer the wavelength of the emitted photon
Examiner Tips and Tricks
Although the difference in energy ΔE = E1 – E2 can be expressed in eV, you need to convert this value in Joules when you are asked to calculate either the frequency or wavelength of the emitted photon.
You are expected to be able to calculate the frequency or the wavelength of a photon, given a specific transition on an energy levels diagram or to identify a specific transition on a given diagram when provided with the value of frequency or wavelength.
You are not expected to know the Bohr formula as given in the worked example - this is just an example of an unfamiliar context you could be given that you have to apply your knowledge to.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
