Determination of Rate Equation (Oxford AQA International A Level (IAL) Chemistry): Revision Note
Exam code: 9622
Concentration-Time Graphs
Using concentration–time graphs
Concentration–time graphs can be used to deduce:
The overall rate of a reaction
The order with respect to an individual reactant
In a zero-order reaction, the concentration of the reactant is inversely proportional to time
This means that the concentration of the reactant decreases with increasing time
The graph is a straight line going down

In a first-order reaction, the concentration of the reactant decreases with time
The graph is a curve going downwards and eventually plateaus

In a second-order reaction, the concentration of the reactant decreases more steeply with time
The concentration of reactant decreases more with increasing time compared to in a first-order reaction
The graph is a steeper curve going downwards

Using initial concentration–time data
Initial concentration–time data can be used to deduce the initial rate of a reaction
The initial rate of a reaction is the rate right at the start of the reaction
This is used because right at the start of the reaction, we know the exact concentration of the reactants used
To calculate the initial rate of reaction:
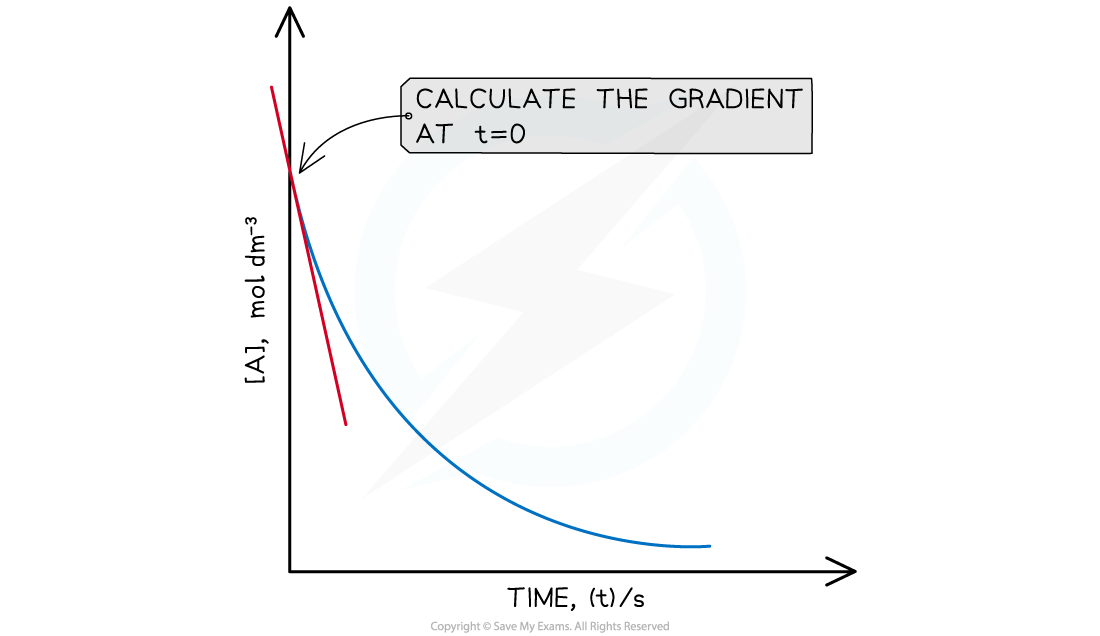
Draw a tangent to the curve at time, t = 0 s
Calculate the gradient of the tangent line
One alternative to this gathers experimental data to determine the order with respect to the reactants in the reaction
The method involves setting up a series of experiments
When carrying out the experiments:
The temperature must remain constant
For each experiment, the concentration of only one reactant is altered
The remaining reactant concentrations remain constant
The experiments are planned so that when the results are collected, they can be used to determine the order with respect to each reactant
For each experiment, a concentration-time graph is drawn
From each graph, the initial rate is calculated by drawing a tangent to the line at t = 0 and calculating the gradient
The gradient at t = 0 is the initial rate for that reaction
General Example
Let's take the following general reaction as an example
2A + B + C → C + D
We need to run a series of experiments at different concentrations of A, B and C, to determine how each affects the initial rate of the reaction
Experiment 1 - use the same concentrations of A, B and C
Experiment 2 - change the concentration of A but keep the concentrations of B and C the same as in experiment 1
Experiment 3 - change the concentration of B but keep the concentrations of A and C the same as in experiment 1
Experiment 4 - change the concentration of C but keep the concentrations of A and B the same as in experiment 1
Plot graphs for each experiment
Draw a tangent at t=0 and calculate the gradient (the initial rate) for each graph
Record the concentrations and initial rates of reaction in a suitable table
Experiment | Initial [A] | Initial [B] | Initial [C] | Initial rate |
|---|---|---|---|---|
1 | 1.5 x 10-3 | 1.5 x 10-3 | 1.5 x 10-3 | 2.1 x 10-3 |
2 | 3.0 x 10-3 | 1.5 x 10-3 | 1.5 x 10-3 | 2.1 x 10-3 |
3 | 1.5 x 10-3 | 3.0 x 10-3 | 1.5 x 10-3 | 4.2 x 10-3 |
4 | 1.5 x 10-3 | 1.5 x 10-3 | 3.0 x 10-3 | 8.4 x 10-3 |
Use these results to determine the order with respect to each reactant
Order with respect to [A]:
Use experiments 1 and 2
[A] doubles
No effect on the intial rate
Therefore, the reaction is zero order with respect to [A]
Order with respect to [B]:
Use experiments 1 and 3
[B] doubles
The intial rate doubles
Therefore, the reaction is first order with respect to [B]
Order with respect to [C]:
Use experiments 1 and 4
[C] doubles
The initial rate increases by a factor of 4
Therefore, the reaction is second order with respect to [C]
Use the orders, to write the rate equation for the reaction:
Rate = k [B] [C]2
Using rate-concentration graphs
The progress of the reaction can be followed by measuring the initial rates of the reaction using various initial concentrations of each reactant
These rates can then be plotted against time in a rate-time graph
In a zero-order reaction, the rate does not depend on the concentration of the reactant
The rate of the reaction therefore remains constant throughout the reaction
The graph is a horizontal line
The rate equation is rate = k

In a first-order reaction, the rate is directly proportional to the concentration of a reactant
The rate of the reaction decreases as the concentration of the reactant decreases when it gets used up during the reaction
The graph is a straight line
The rate equation is rate = k [A]

In a second-order reaction, the rate is directly proportional to the square of concentration of a reactant
The rate of the reaction decreases more as the concentration of the reactant decreases when it gets used up during the reaction
The graph is a curved line
The rate equation is rate = k [A]2

Examiner Tips and Tricks
Careful - sometimes when asked to complete calculations for k, the exam question will give you a graph which demonstrates the order of one of the reactants, as well as tabulated data to determine the order for the other reactants. Do not ignore the graph.
Rates & Reaction Mechanisms
The reaction mechanism of a reaction describes how many steps are involved in the making and breaking of bonds during a chemical reaction
It is the slowest step in a reaction and includes the reactants that have an impact on the reaction rate when their concentrations are changed
Therefore, all reactants that appear in the rate equation will also appear in the rate-determining step
This means that zero-order reactants and intermediates will not be present in the rate-determining step
Predicting the reaction mechanism
The overall reaction equation and rate equation can be used to predict a possible reaction mechanism of a reaction
For example, nitrogen dioxide (NO2) and carbon monoxide (CO) react to form nitrogen monoxide (NO) and carbon dioxide (CO2)
The overall reaction equation is:
NO2 (g) + CO (g) → NO (g) + CO2 (g)
The rate equation is:
Rate = k [NO2]2
From the rate equation, it can be concluded that the reaction is zero order with respect to CO (g) and second order with respect to NO2 (g)
This means that there are two molecules of NO2 (g) involved in the rate-determining step
A possible reaction mechanism could therefore be:
Step 1:
2NO2 (g) → NO (g) + NO3 (g) slow (rate-determining step)
Step 2:
NO3 (g) + CO (g) → NO2 (g) + CO2 (g) fast
Overall:
2NO2 (g) +NO3(g)+ CO (g) → NO (g) +NO3(g)+NO2(g)+ CO2 (g)Which simplifies to NO2 (g) + CO (g) → NO (g) + CO2 (g)
Predicting the reaction order & deducing the rate equation
The order of a reactant and thus the rate equation can be deduced from a reaction mechanism given that the rate-determining step is known
For example, the reaction of nitrogen oxide (NO) with hydrogen (H2) to form nitrogen (N2) and water
2NO (g) + 2H2 (g) → N2 (g) + 2H2O (l)
The reaction mechanism for this reaction is:
Step 1:
NO (g) + NO (g) → N2O2 (g) fast
Step 2:
N2O2 (g) + H2 (g) → H2O (l) + N2O (g) slow (rate-determining step)
Step 3:
N2O (g) + H2 (g) → N2 (g) + H2O (l) fast
The second step in this reaction mechanism is the rate-determining step
The rate-determining step consists of:
N2O2 which is formed from the reaction of two NO molecules
One H2 molecule
The reaction is, therefore, second order with respect to NO and first order with respect to H2
So, the rate equation becomes:
Rate = k [NO]2 [H2]
The reaction is, therefore, third-order overall
Identifying the rate-determining step
The rate-determining step can be identified from a rate equation given that the reaction mechanism is known
For example, propane (CH3CH2CH3) undergoes bromination under alkaline solutions
The overall reaction is:
CH3CH2CH3 + Br2 + OH- → CH3CH2CH2Br + H2O + Br-
The reaction mechanism is:

The rate equation is:
Rate = k [CH3CH2CH3] [OH-]
From the rate equation, it can be deduced that only CH3COCH3 and OH- are involved in the rate-determining step and not bromine (Br2)
CH3COCH3 and OH- are only involved in step 1
Therefore, the rate-determining step is step 1 of the reaction mechanism
Identifying intermediates & catalyst
When a rate equation includes a species that is not part of the chemical reaction equation then this species is a catalyst
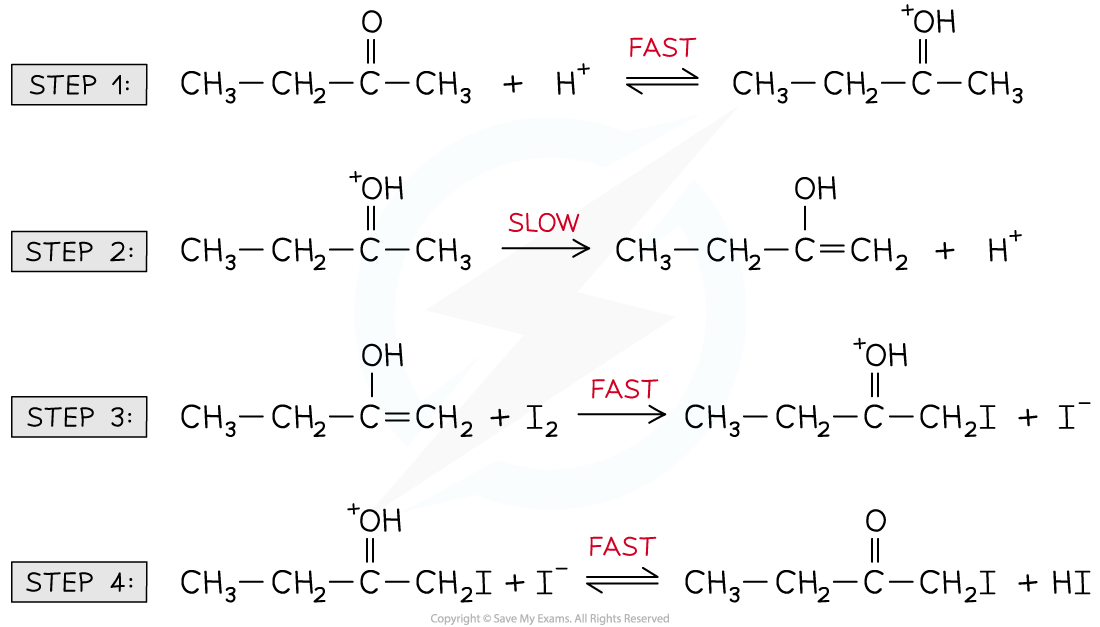
For example, the halogenation of butanone under acidic conditions
The reaction mechanism is:
CH3CH2COCH3 + I2 CH3CH2COCH2I + HI
The reaction mechanism is:
The rate equation is:
Rate = k [CH3CH2COCH3] [H+]
The H+ is not present in the chemical reaction equation but does appear in the rate equation
H+ must therefore be a catalyst
Furthermore, the rate equation suggests that CH3CH2COCH3 and H+ must be involved in the rate-determining (slowest) step
The CH3CH2COCH3 and H+ appear in the rate-determining step in the form of an intermediate (which is a combination of the two species)


Unlock more, it's free!
Did this page help you?