Kp Calculations (Oxford AQA International A Level (IAL) Chemistry): Revision Note
Exam code: 9622
Partial Pressure
For reactions involving mixtures of gases, the equilibrium constant Kp is used
This is because it is easier to measure the pressure than the concentration for gases
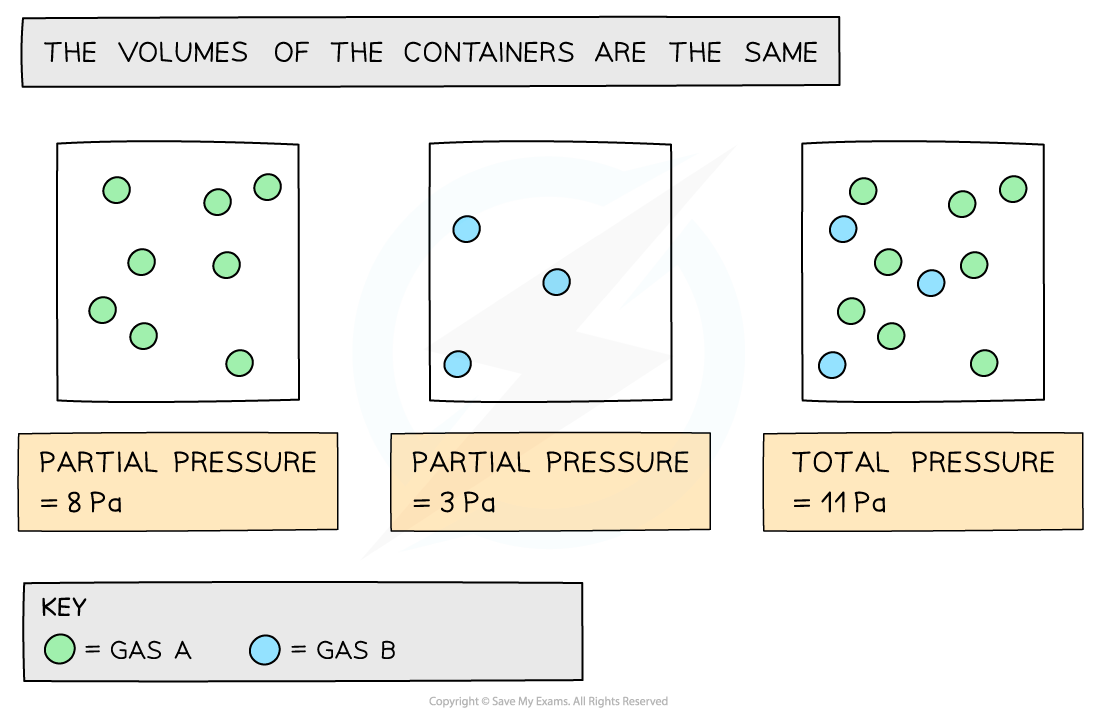
The partial pressure of a gas is the pressure that the gas would have if it was in the container all by itself
The total pressure is the sum of all the partial pressures:
Ptotal = PA + PB + PC + .......
Where:
Ptotal = total pressure
PA, PB, PC = partial pressures
How partial pressures contribute to total pressure
Mole fraction
The mole fraction of a gas is the ratio of moles of a particular gas to the total number of moles of gas present
This equation can be used to calculate the partial pressure of each gas:
Examiner Tips and Tricks
You can check that your mole fractions and partial pressures are correct:
The sum of the mole fractions should add up to 1
The sum of the partial pressures should add up to the total pressure
Worked Example
A sample of 0.25 mole of nitrogen and 0.75 mole of hydrogen were reacted together to form ammonia. The equilibrium amount of nitrogen was 0.16 mole at a pressure of 75 kPa.
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Calculate the mole fractions of nitrogen, hydrogen and ammonia.
Calculate the partial pressures of nitrogen, hydrogen and ammonia.
Answer:
The mole fractions of nitrogen, hydrogen and ammonia:
Complete an ICE table:
N2 (g)
3H2 (g)
2NH3 (g)
Initial
0.25 mol
0.75 mol
0.00 mol
Change
0.25 - 0.16
= -0.09
-3 x 0.09
= -0.27
+2 x 0.09
= +0.18
Equilibrium
0.16 mol
0.75 - 0.27
= 0.48 mol
0.18 mol
Calculate the total number of moles:
0.16 + 0.48 + 0.18 = 0.82
Calculate the mole fractions:
N2 (g) = 0.16 / 0.82 = 0.195
H2 (g) = 0.48 / 0.82 = 0.585
NH3 (g) = 0.18 / 0.82 = 0.220
The partial pressures of nitrogen, hydrogen and ammonia:
Partial pressure = mole fraction x total pressure
N2 (g) = 0.195 x 75 = 14.625 kPa
H2 (g) = 0.585 x 75 = 43.875 kPa
NH3 (g) = 0.220 x 75 = 16.5 kPa
Kp Calculations
Kp calculations are a step-by-step process
Some questions may give the equilibrium partial pressure of all the gases
This means that the only remaining steps are:
Write the Kp expression
Calculate the value of Kp
Worked Example
The equilibrium between sulfur dioxide, oxygen and sulfur trioxide is as follows:
2SO2 (g) + O2 (g) 2SO3 (g)
At constant temperature, the equilibrium partial pressures are:
SO2 = 1.0 x 106 Pa
O2 = 7.0 x 106 Pa
SO3 = 8.0 x 106 Pa
Calculate the value of Kp for this reaction.
Answer:
Step 1: Write the equilibrium constant for the reaction in terms of partial pressures:
Kp =
Step 2: Substitute the equilibrium concentrations into the expression:
Kp =
Kp = 9.1 x 10–6
Step 3: Deduce the correct units of Kp:
Kp =
So, the units of Kp are Pa-1
Therefore, Kp = 9.1 x 10-6 Pa-1
Some questions give the number of moles of gases present and the total pressure
So, the steps to complete these problems are:
Calculate the mole fractions
Calculate the partial pressures
Write the Kp expression
Calculate the value of Kp
Worked Example
The equilibrium between hydrogen, iodine and hydrogen iodide is as follows:
H2 (g) + I2 (g) 2HI (g)
At constant temperature, the equilibrium moles are:
H2 = 1.71 x 10–3
I2 = 2.91 x 10–3
HI = 1.65 x 10–2
The total pressure is 100 kPa.
Calculate the value of Kp for this reaction.
Answer:
Step 1: Calculate the total number of moles:
Total number of moles = 1.71 x 10-3 + 2.91 x 10-3 + 1.65 x 10-2
Total number of moles = 2.112 x 10-2
Step 2: Calculate the mole fraction of each gas:
H2 =
= 0.0810
I2 =
= 0.1378
HI =
= 0.7813
Step 3: Calculate the partial pressure of each gas:
H2 = 0.0810 x 100 = 8.10 kPa
I2 = 0.1378 x 100 = 13.78 kPa
HI = 0.7813 x 100 = 78.13 kPa
Step 4: Write the equilibrium constant in terms of partial pressure:
Kp =
Step 5: Substitute the values into the equilibrium expression:
Kp =
Kp = 54.7
Step 6: Deduce the correct units for Kp:
Kp =
All units cancel out
Therefore, Kp = 54.7
A full Kp question will give:
Initial number of moles of the gases present
The equilibrium number of moles of at least one of the gases present
The total pressure
So, the steps to complete these problems are:
Determine the number of equilibrium moles of all gases
Calculate the mole fractions
Calculate the partial pressures
Write the Kp expression
Calculate the value of Kp
Worked Example
Hydrogen and bromine were mixed in a flask in a 1 : 1 ratio and allowed to reach equilibrium at 450 K. When equilibrium had been achieved the total pressure in the flask was 140 kPa and the mole fraction of bromine was 0.35.
The equation for the reaction is:
H2 (g) + Br2 (g) ⇌ 2HBr (g)
Determine the partial pressures for each gas at equilibrium and the value of Kp.
Answer:
H2 and Br2 are in a 1 : 1 ratio
So, the mole fraction of Br2 = the mole fraction of H2
Mole fraction of hydrogen = 0.35
The mole fraction of hydrogen bromide is the remainder of the mole fraction
This is because the total mole fraction adds up to 1
So, the mole fraction of HBr = 1 - the mole fraction of H2 - the mole fraction of Br2
Mole fraction of hydrogen bromide = 1 - 0.35 - 0.35 = 0.3
The partial pressures are calculated by mole fraction x total pressure:
Partial pressure H2 = 0.35 x 140 = 49 kPa
Partial pressure Br2 = 0.35 x 140 = 49 kPa
Partial pressure HBr = 0.30 x 140 = 42 kPa
The Kp expression is:
Kp =
The value of Kp is:
Kp =
= 0.735
There are no units for this Kp value
The kPa2 term on top is cancelled by the kPa x kPa on the bottom
Examiner Tips and Tricks
You need to be able to start from initial moles to calculate equilibrium moles and continue to calculate Kp values.
You also need to be able to work backwards to deduce partial pressures and equilibrium moles from given information.

Unlock more, it's free!
Did this page help you?