Applications of Hess’s Law (Oxford AQA International A Level (IAL) Chemistry): Revision Note
Exam code: 9622
Hess's Law Calculations
Hess’s Law states that:
"The total enthalpy change in a chemical reaction is independent of the route taken."
This means that whether the reaction takes place in one or two steps, the total enthalpy change of the reaction will still be the same
Illustration of Hess's Law
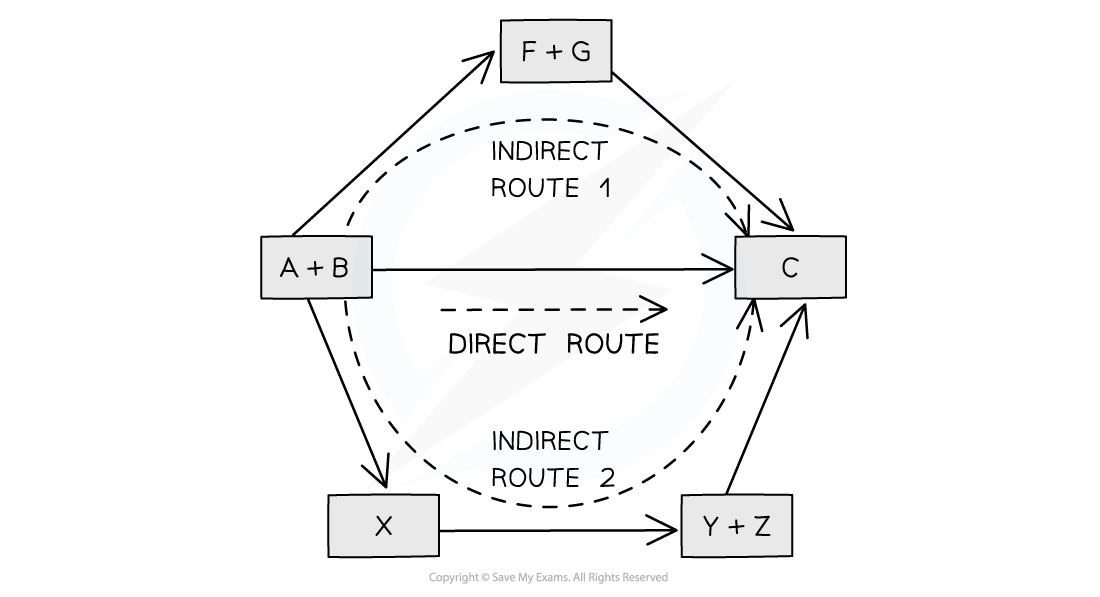
Hess’ Law is used to calculate enthalpy changes which can’t be found experimentally using calorimetry, such as:
3C (s) + 4H2 (g) → C3H8 (g)
ΔHf (propane) can’t be found experimentally as hydrogen and carbon don’t react under standard conditions
Calculating ΔHf from ΔHc using Hess’s Law energy cycles
It can be difficult to find the enthalpy change of formation of compounds experimentally
However, many enthalpy changes of combustion can be measured experimentally so these can be used to find the enthalpy of formation
To do this, we follow these steps:
Write the equation for the formation of the compound
Write the combustion products below the equation
Draw downward pointing arrows from each substance to its combustion products
Write values on the arrows and multiply by the number of moles
In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction
Worked Example
Using the data provided, calculate the standard enthalpy change of formation, ΔHf, of propanone.
3C (s) + 3H2 (g) + ½ O2 (g) → CH3COCH3 (l)
Substance | C (s) | H2 (g) | CH3COCH3 (l) |
|---|---|---|---|
∆HCө / kJ mol–1 | -394 | -286 | -1821 |
Answer:
Step 1: Write the balanced equation

Step 2:Write the combustion products below the equation

Step 3: Draw downward pointing arrows from each substance to its combustion product

Step 4: Write the appropriate values on the arrows and multiply by the number of moles

Step 5: In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction

ΔHfө = -1182 - 858 + 1821 = -219 kJ mol-1
The sign on -1821 needs reversing as the cycle goes in the opposite direction to the arrow pointing to the combustion products
Calculating ΔHr from ΔHf using Hess’s Law energy cycles
Knowing the enthalpy change of formation, ΔHf, allows us to determine the overall enthalpy change of a reaction, ΔHr
To do this, we follow these steps:
Write the equation for the reaction
Write the elements with the correct number of moles and state symbols underneath
Draw upwards pointing arrows to each compound
Write the appropriate values on the arrows and multiply by the number of moles
In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction
Worked Example
Use the information in the table to calculate the enthalpy change for this reaction:
NH4NO3 (s) + ½C (s) → N2 (g) + 2H2O (g) + ½CO2 (g)
Substance | C (s) | N2 (g) | H2O(g) | CO2 (g) | NH4NO3 (s) |
|---|---|---|---|---|---|
∆Hfө / kJ mol–1 | 0 | 0 | –242 | –394 | –365 |
Answer:
Step 1: Write the balanced equation

Step 2: Write the elements with the correct number of moles and state symbols underneath

Step 3: Draw upwards pointing arrows to each compound

Step 4: Write the appropriate values on the arrows and multiply by the number of moles

Step 5: In a cycle, go from the reactants to the products, changing the sign of the value if the arrow points in the opposite direction

ΔHrө = +365 - 484 - 197 = -316 kJ mol-1
The sign on -365 needs reversing as the cycle goes in the opposite direction to the arrow pointing upwards
There is no need to draw arrows from the elements to carbon and nitrogen as ΔHfө is 0 for elements

Unlock more, it's free!
Did this page help you?