Volumetric Solutions & Analysis (Oxford AQA International A Level (IAL) Chemistry): Revision Note
Exam code: 9622
Required Practical 1: Volumetric Solutions & Acid-Base Titration
Required practical 1 is split into:
Part A: Make up a volumetric solution
Part B: Carry out a simple acid-base titration
Part A: Make up a volumetric solution
Objective
To prepare 250 cm3 of 0.100 mol dm-3 sodium hydrogensulfate solution, NaHSO4 (aq).
Apparatus
Weighing bottle
250 cm3 volumetric flask
Solid sodium hydrogensulfate, NaHSO4 (s)
Mr = 120.1 g mol-1
Filter funnel
Spatula
Deionised / distilled water
250 cm3 beaker
Glass rod
Digital mass balance (reading to 2 or 3 decimal places)
Method
Calculate the mass of solid sodium hydrogensulfate required to make 250 cm3 of a 0.100 mol dm-3 solution
1 mol dm-3 NaHSO4 = 120.1 g in 1 dm3
0.100 mol dm-3 NaHSO4 = 12.01 g in 1 dm3
0.100 mol dm-3 NaHSO4 = 3.0225 g in 250 cm3
Measure out 3.0225 g of solid sodium hydrogensulfate into the weighing bottle
The exact mass needs to be recorded because it is not possible to measure to this precision with the mass balances for this experiment
Transfer the solid sodium hydrogensulfate from the weighing bottle to the beaker
Rinse the weighing bottle with deionised water and add the washings to the beaker
Add 100 cm3 of deionised water and stir until all of the solid dissolves
Transfer the sodium hydrogen carbonate solution from the beaker to the volumetric flask
Rinse the beaker and glass rod with deionised water and add the washings to the volumetric flask
Make the volumetric flask up to the graduation mark
Insert the stopper and shake / invert the volumetric flask to mix the contents
Calculate the exact concentration of the sodium hydrogensulfate solution in mol dm-3
Diagram
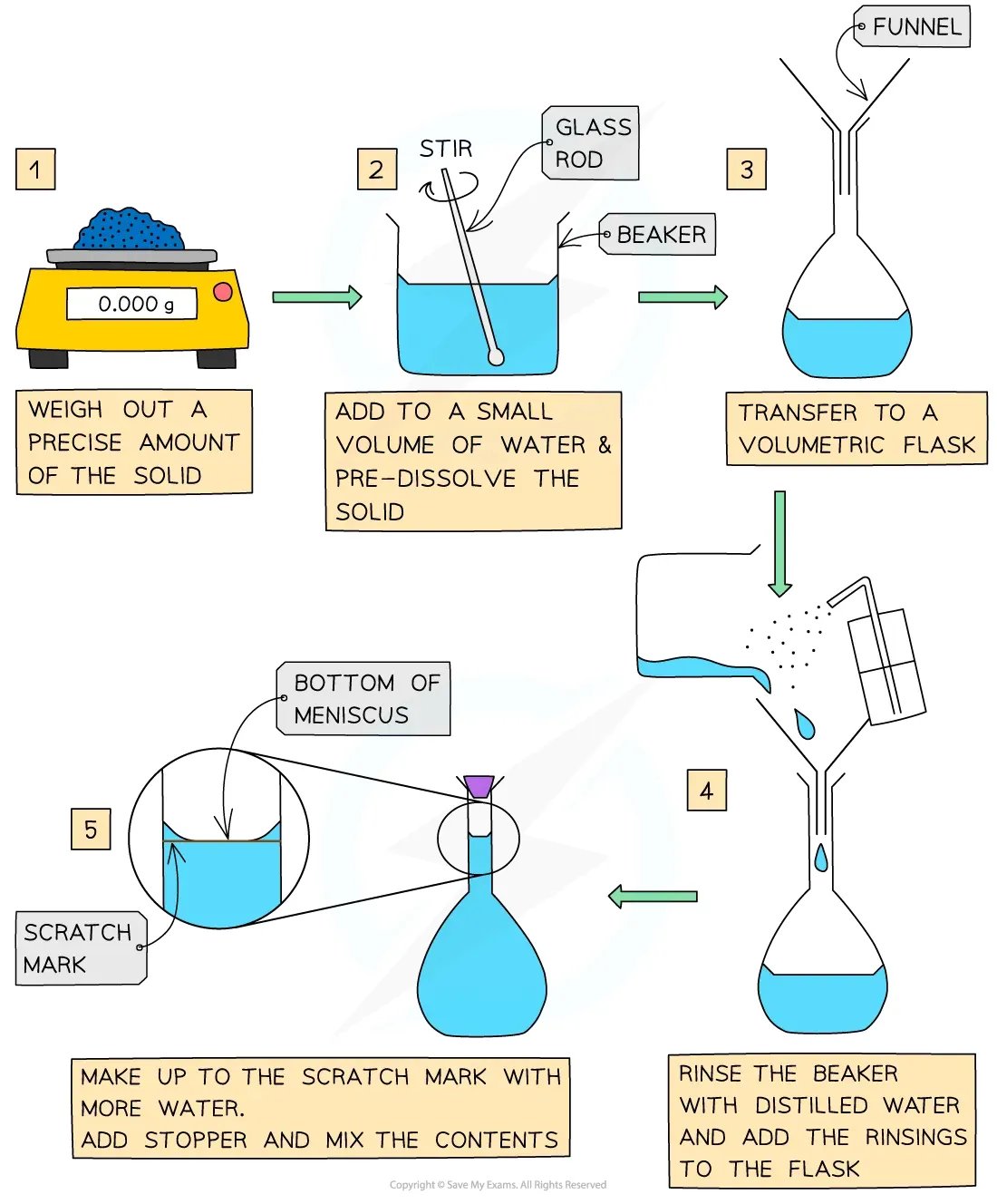
Practical Tip
The most common source of error in the preparation of a standard solution is the loss of solid, when it is transferred from one vessel to another
Results
The only result for this part of the required practical is the mass of sodium hydrogensulfate
e.g. mass of sodium hydrogensulfate = 3.02 g
Remember: The exact mass of sodium hydrogensulfate is determined by the number of decimal places on the mass balance
Evaluation
The exact concentration of the sodium hydrogensulfate solution is:
Mass of NaHSO4 = 3.02g
Moles of NaHSO4 =
= 0.0251
Concentration =
Concentration =
= 0.1004 mol dm-3
Worked Example
Calculate the mass of sodium hydrogensulfate monohydrate, NaHSO4.H2O, required to prepare 250 cm3 of a 0.050 mol dm-3 solution.
Give your answer to 3 significant figures.
Answer:
Calculate the number of moles of NaHSO4.H2O needed from the concentration and volume:
n(NaHSO4.H2O) = concentration (mol dm-3) x volume (dm3)
n(NaHSO4.H2O) = 0.050 mol dm–3 x 0.250 dm3
n(NaHSO4.H2O) = 0.0125 mol
Calculate the molar mass of NaHSO4.H2O:
Mr = 23.0 + (3 x 1.0) + 32.1 + (5 x 16.0) = 138.1 g mol–1
Calculate the mass of NaHSO4.H2O required:
mass = moles x molar mass
mass = 0.0125 mol x 138.1 g mol–1 =1.73 g
Part B: Carry Out a Simple Acid-Base Titration
Objective
To determine the concentration of a solution of sodium hydroxide by titration using a sodium hydrogensulfate solution that has a known concentration.
Apparatus
Burette, stand and clamp
25 cm3 volumetric pipette with pipette bulb / filler
250 cm3 conical flasks
Funnel
Deionised / distilled water wash bottle
Phenolphthalein indicator
150 cm3 sodium hydrogensulfate (standard) solution
150 cm3 sodium hydroxide (unknown concentration) solution
Method
Rinse the burette with the sodium hydrogensulfate standard solution
Then fill the burette with the sodium hydrogensulfate standard solution
Rinse a 250 cm3 conical flask with deionised / distilled water
Rinse the 25 cm3 volumetric pipette with the sodium hydroxide solution
Use the volumetric pipette to transfer exactly 25.0 cm3 of sodium hydroxide solution into the rinsed 250 cm3 conical flask
Add two to three drops of phenolphthalein indicator to the solution in the conical flask
Construct a results table
Record the initial burette reading to the appropriate precision
Titrate the contents of the conical flask by adding sodium hydrogensulfate solution to it from the burette
Add the sodium hydrogensulfate solution slowly with swirling to mix the solution
Add the sodium hydrogensulfate solution dropwise near the end-point
At the end-point the indicator undergoes a definite colour change
Record the final burette reading in your results table
In your results table, calculate / record the volume of sodium hydrogensulfate solution used
Repeat the titration until you obtain two results which are concordant
Concordant results are within 0.1 cm3
You should normally carry out at least three titrations
Record all of the results that you obtain.
Calculate / record the mean (average) volume of sodium hydrogensulfate solution used in the
titration
Show your working
Use your results to calculate the concentration of the sodium hydroxide
Show your working
Diagram
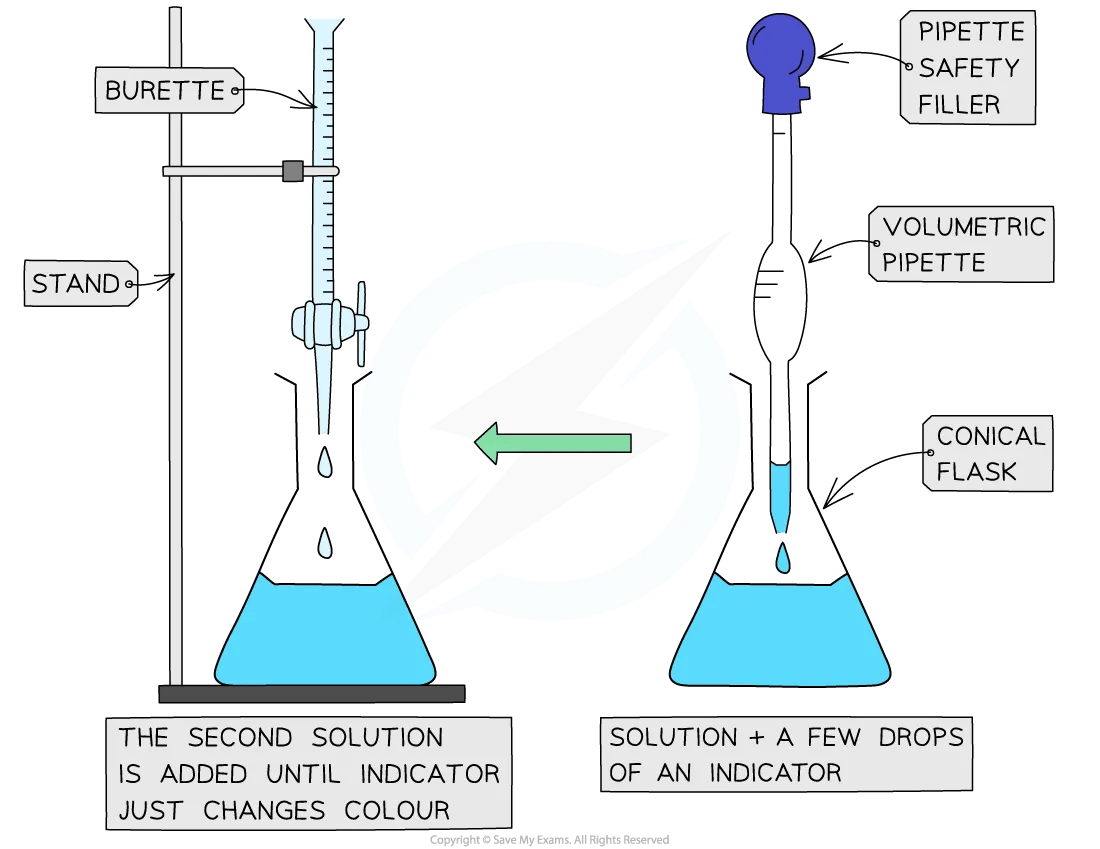
Practical Tip
Completing a rough titration first helps to determine the approximate end-point of the titration
This also allows you to run the burette until 2 - 3 cm3 before the end-point and slowly add the sodium hydrogensulfate solution dropwise
Results
Record your results for each test carefully in a suitable table like the one below:
Rough | Run 1 | Run 2 | Run 3 | |
|---|---|---|---|---|
Initial burette reading (cm3) | ||||
Final burette reading (cm3) | ||||
Titre volume (cm3) |
Evaluation
Identify the concordant results
Remember: Concordant results are within 0.1 cm3
Calculate the mean average titre
Calculate the moles of the chemical with the known concentration
In this case, this is the sodium hydrogensulfate
Use the balanced chemical equation to deduce the moles of the chemical with the unknown concentration
In this case, this is the sodium hydroxide
Calculate the concentration of the chemical with the unknown concentration
Careful: This may require the volume converting from cm3 to dm3
Worked Example
A sodium hydroxide solution of unknown concentration was titrated against a 0.100 mol dm-3 solution of sodium hydrogen sulfate.
NaHSO4 (aq) + NaOH (aq) → Na2SO4 (aq) + H2O (l)
The titration results are shown in the table below.
Rough | Run 1 | Run 2 | Run 3 | |
|---|---|---|---|---|
Initial burette reading (cm3) | 0.00 | 0.00 | 0.10 | 0.10 |
Final burette reading (cm3) | 26.10 | 25.10 | 24.85 | 25.10 |
Titre volume (cm3) | 26.10 | 25.10 | 24.75 | 25.00 |
Determine the concentration of the sodium hydroxide.
Answer:
Identify the concordant results:
The concordant results are runs 1 and 3
25.10 cm3 and 25.00 cm3 are within 0.1 cm3
Calculate the average titre:
Average titre =
= 25.05 cm3
Calculate the moles of sodium hydrogensulfate:
n(NaHSO4) = concentration x volume
n(NaHSO4) = 0.100 mol dm-3 x
dm3
n(NaHSO4) = 0.002505 moles
Deduce the moles of sodium hydroxide:
From the balanced equation, one mole of sodium hydroxide reacts with 1 mole of sodium hydrogensulfate
i.e. n(NaHSO4) = n(NaOH)
Therefore, n(NaOH) = 0.002505 moles
Convert the volume of sodium hydroxide solution from cm3 to dm3
= 0.025 dm3
Calculate the concentration of the unknown sodium hydroxide solution:
[NaOH (aq)] =
[NaOH (aq)] =
[NaOH (aq)] = 0.1002 mol dm-3

Unlock more, it's free!
Did this page help you?