The Mole & The Avogadro Constant (Oxford AQA International A Level (IAL) Chemistry): Revision Note
Exam code: 9622
The Mole & the Avogadro Constant
The Avogadro constant (NA or L) is the number of particles equivalent to the relative atomic mass or molecular mass of a substance
The Avogadro constant applies to atoms, molecules, ions and electrons
The value of NA is 6.02 x 1023 g mol-1
The mass of a substance with this number of particles is called a mole (mol)
The mass of a substance containing the same number of fundamental units as there are atoms in exactly 12.00 g of 12C
One mole of any element is equal to the relative atomic mass of that element in grams
One mole of carbon, that is if you had 6.02 x 1023 atoms of carbon in your hand, would have a mass of 12 g
One mole of water would have a mass of (2 x 1 + 16) = 18 g
The number of moles or particles can be calculated easily using a formula triangle:
Formula triangle diagram linking moles, particles and Avogadro's constant

Worked Example
Determine the number of atoms, molecules and the relative mass of 1 mole of:
Na
H2
NaCl
Answer 1
The relative atomic mass of Na is 23.0
Therefore, 1 mol of Na has a mass of 23.0 g mol-1
1 mol of Na will contain 6.02 x 1023 atoms of Na (Avogadro’s constant)
Answer 2
The relative atomic mass of H is 1.0
Since there are 2 H atoms in H2, the mass of 1 mol of H2 is (2 x 1.0) = 2.0 g mol-1
1 mol of H2 will contain 6.02 x 1023 molecules of H2
Since there are 2 H atoms in H2, 1 mol of H2 will contain 2 x 6.02 x 1023 = 1.204 x 1024 H atoms
Answer 3
The relative atomic mass of Na and Cl is 23.0 and 35.5 respectively
Therefore, 1 mol of NaCl has a mass of (23.0 + 35.5) = 58.5 g mol-1
1 mol of NaCl will contain 6.02 x 1023 molecules of NaCl
Since there is one Na and one Cl atom in NaCl, 1 mol of NaCl will contain 2 x 6.02 x 1023 = 1.204 x 1024 atoms in total
1 mole of | Number of atoms | Number of molecules | Relative mass |
|---|---|---|---|
Na | 6.02 x 1023 | - | 23.0 |
H2 | 1.204 x 1024 | 6.02 x 1023 | 2.0 |
NaCl | 1.204 x 1024 | 6.02 x 1023 | 58.5 |
Examiner Tips and Tricks
You only have to be able to work with Avogadro's constant, you will not be expected to recall the value.
Other mole calculations
The mole is an integral part of other chemical calculations:
Moles, mass and Mr
The number of moles of any chemical can be calculated from the mass of the chemical combined with the Periodic Table
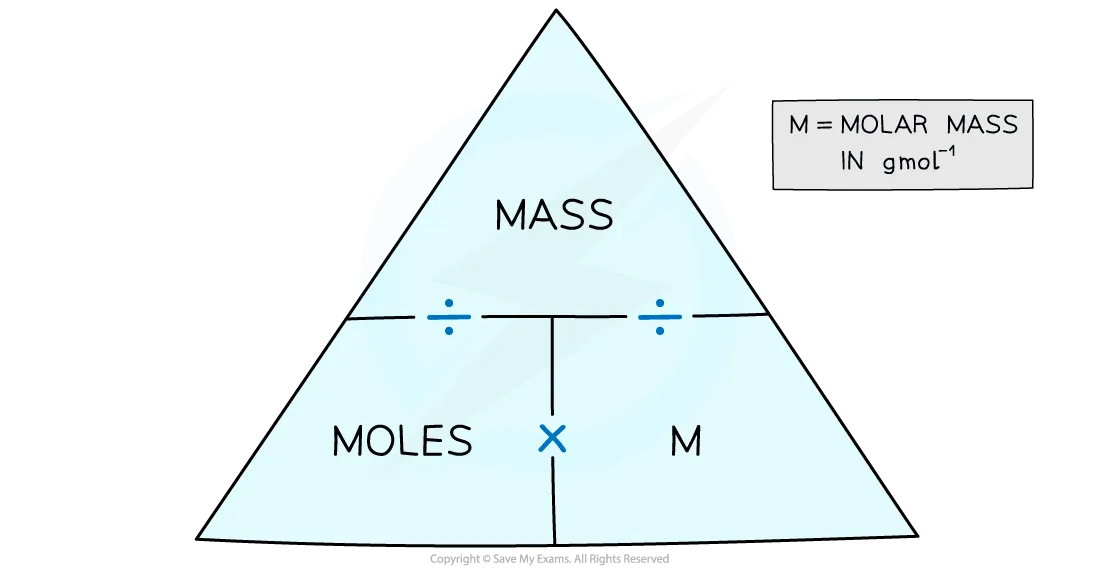
With this information, the number of moles can be calculated by using a formula triangle:
Formula triangle diagram linking moles, mass and molar mass
Worked Example
What is the mass of 0.250 moles of zinc?
How many moles are in 2.64 g of sucrose, C12H11O22 (Mr = 342.3)?
Answers:
0.250 moles of zinc
From the periodic table, the relative atomic / molar mass of Zn is 65.4 g mol-1
Mass = moles x molar mass
Mass = 0.250 mol x 65.4 g mol-1 = 16.4 g
2.64 g of sucrose:
The molar mass of sucrose is 342.3 g mol-1
Moles = mass ÷ molar mass
Moles = 2.64 g ÷ 342.3 g mol-1 = 7.71 x 10-3 mol
Moles, concentration and volume
The number of moles of any chemical can be calculated using the concentration and volume of the chemical
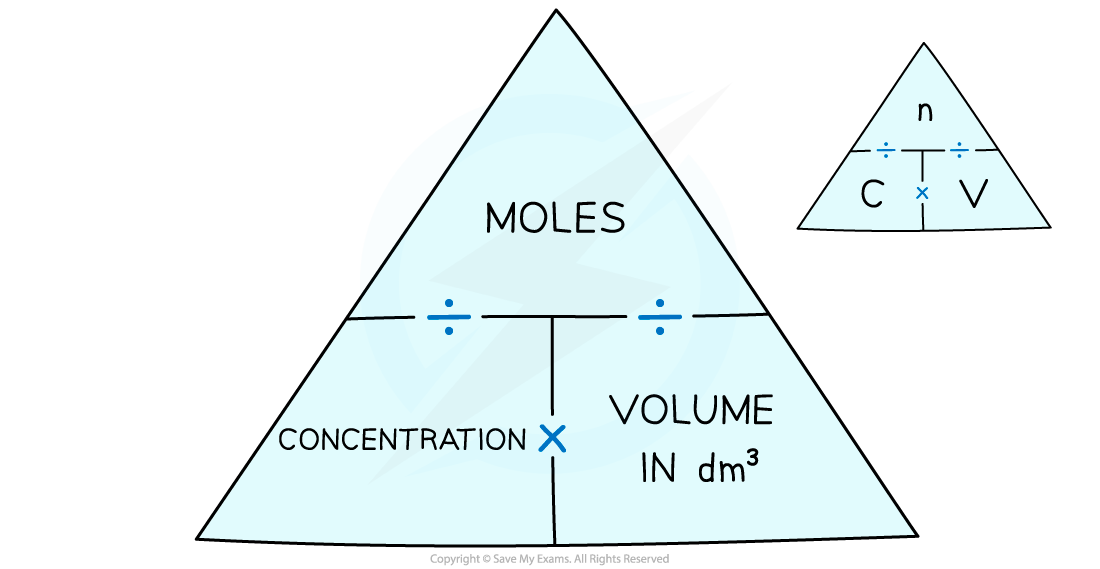
With this information, the number of moles can be calculated by using a formula triangle:
Formula triangle diagram linking moles, concentration and volume
A common application of the moles = concentration x volume formula is titration calculations, e.g. Required Practical 1
Worked Example
Calculate the concentration, mol dm-3, of the solution formed when 80.0 g of sodium hydroxide is dissolved in 500 cm3 of water.
Answer:
The relative formula mass of NaOH is:
23.0 + 16.0 + 1.0 = 40.0 g mol-1
So, 80 g of sodium hydroxide is:
Moles =
Moles =
= 2 moles
The volume in dm3 is:
= 0.5 dm3
The concentration in mol dm-3 is:
Concentration =
Concentration =
= 4 mol dm-3
Examiner Tips and Tricks
Make sure that you use the correct units for moles, concentration, volume calculations.
These calculations have units of mol dm-3 for concentration and dm3 for volume but questions will typically use cm3.

Unlock more, it's free!
Was this revision note helpful?
