Proteins (Oxford AQA International A Level (IAL) Chemistry) : Revision Note
Proteins
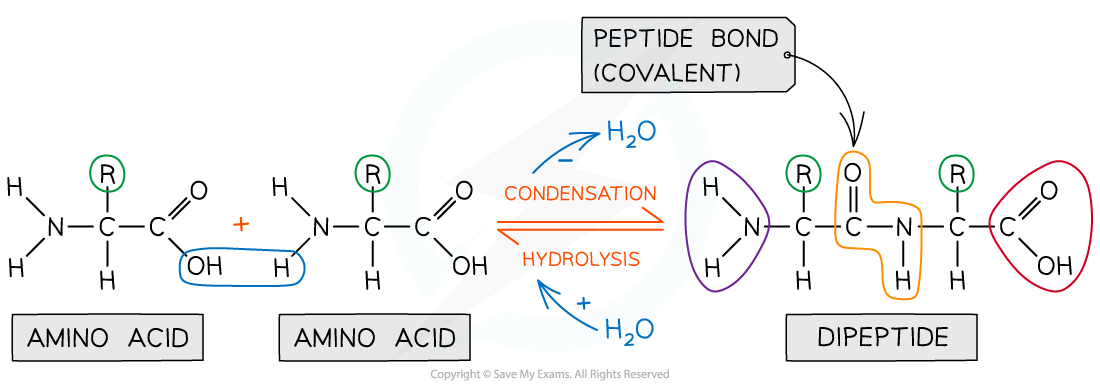
Each amino acid contains an amine (-NH2) and carboxylic acid (-COOH) group
The -NH2 group of one amino acid can react with the -COOH group of another amino acid in a condensation reaction to form a dipeptide
The new amide bond between two amino acids is also called a peptide link or peptide bond
This is a condensation reaction so a small molecule is eliminated (H2O)
The dipeptide still contains an -NH2 and -COOH group at each end of the molecule which can again participate in a condensation reaction to form a tripeptide
Formation of a dipeptide and tripeptide

Polypeptides
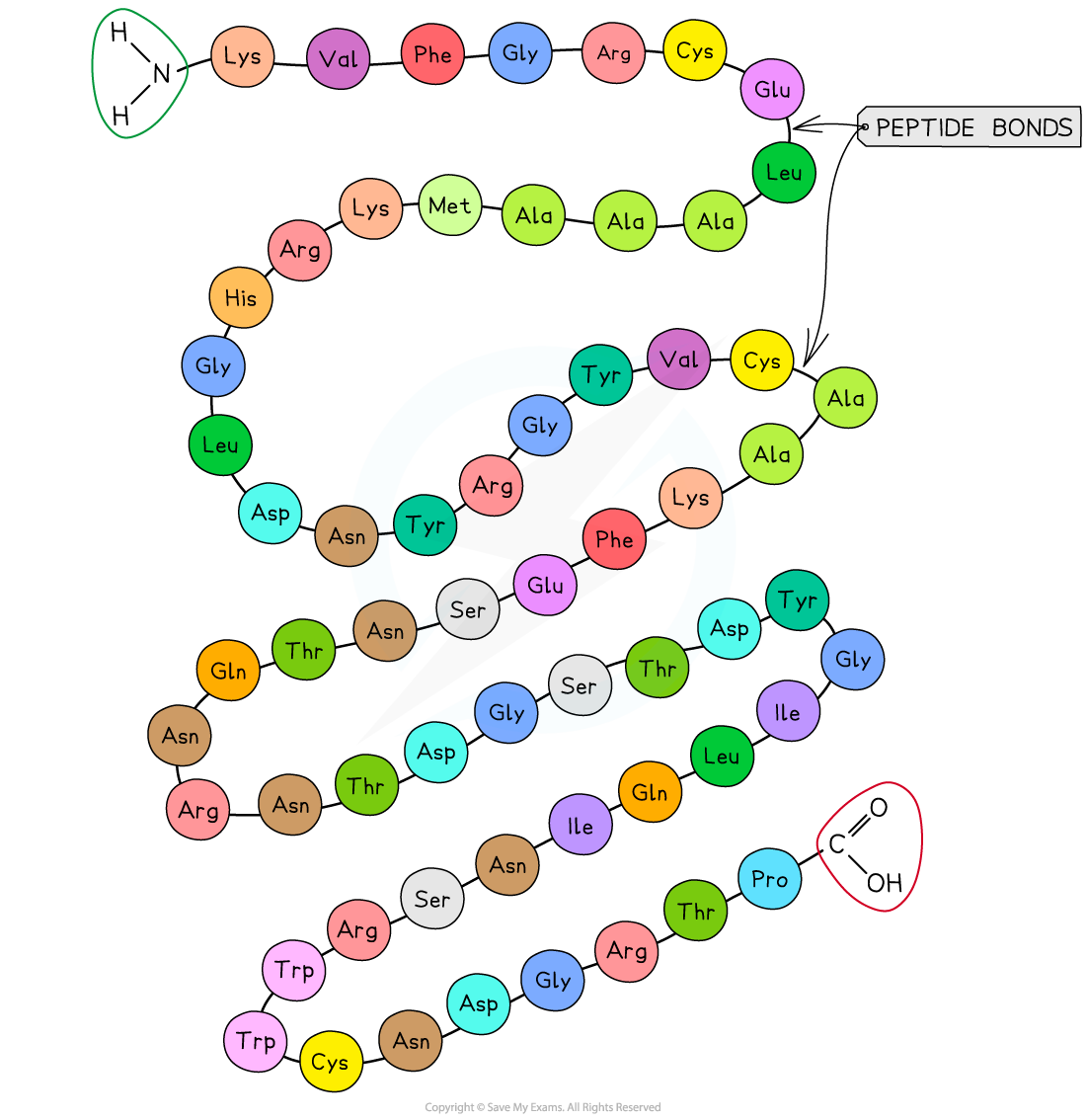
A polypeptide is formed when many amino acids join together to form a long chain of molecules
The structure of a polypeptide

The structure of proteins
There are three main structures of proteins:
Primary
Secondary
Tertiary
Primary
The sequence of amino acids bonded by covalent peptide bonds is the primary structure of a protein
The primary structure is specific for each protein (one alteration in the sequence of amino acids can affect the function of the protein)
The structure is held together by covalent bonding
Secondary
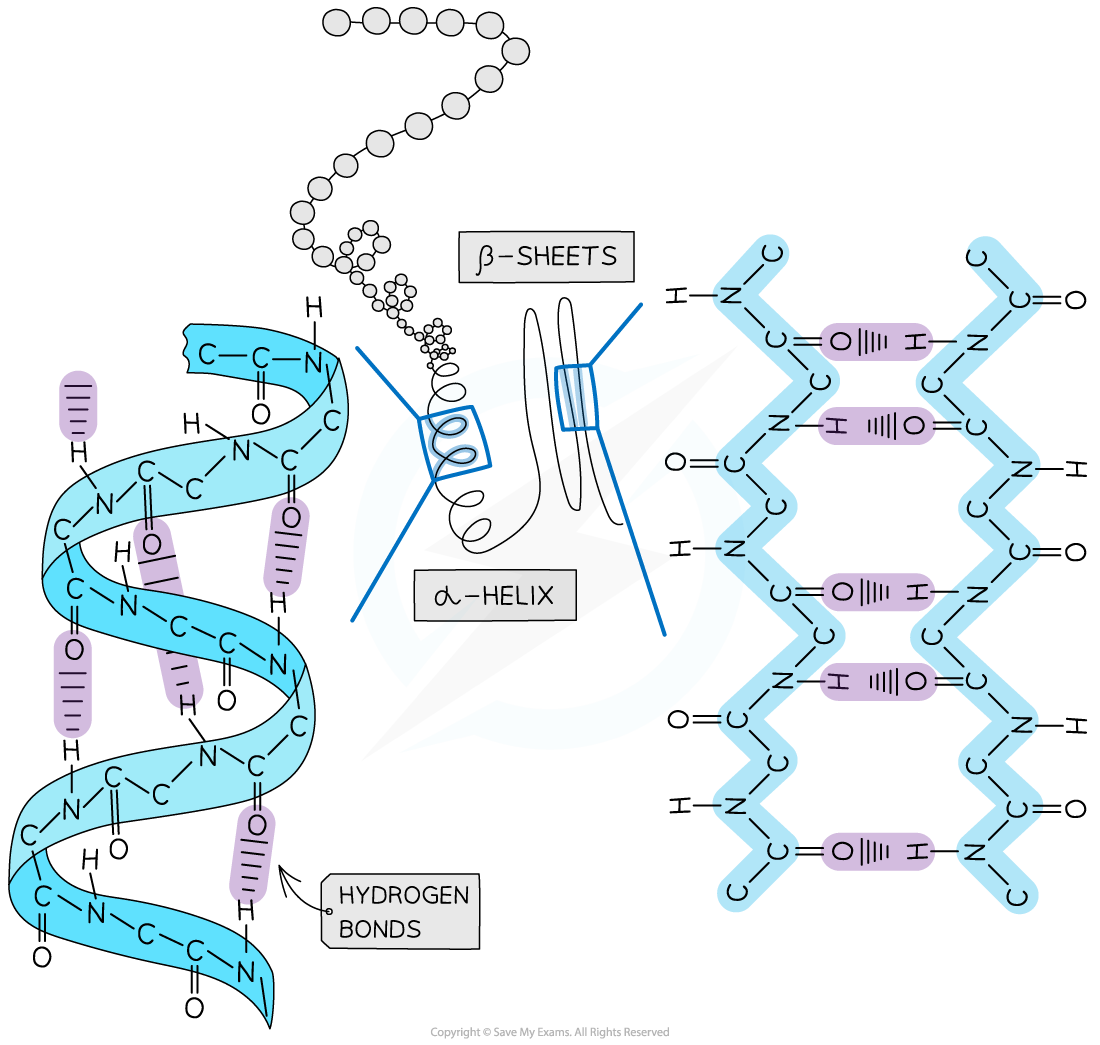
The secondary structure of a protein occurs when the weak negatively charged nitrogen and oxygen atoms interact with the weak positively charged hydrogen atoms to form hydrogen bonds
There are two shapes that can form within proteins due to the hydrogen bonds:
α-helix
β-pleated sheet
The α-helix shape occurs when the hydrogen bonds form between every fourth peptide bond (between the oxygen of the carboxyl group and the hydrogen of the amine group)
The β-pleated sheet shape forms when the protein folds so that two parts of the polypeptide chain are parallel to each other enabling hydrogen bonds to form between parallel peptide bonds
Most fibrous proteins have secondary structures (e.g. collagen and keratin)
The secondary structure only relates to hydrogen bonds forming between the amino group and the carboxyl group (the ‘protein backbone’)
The structures of secondary proteins
Tertiary
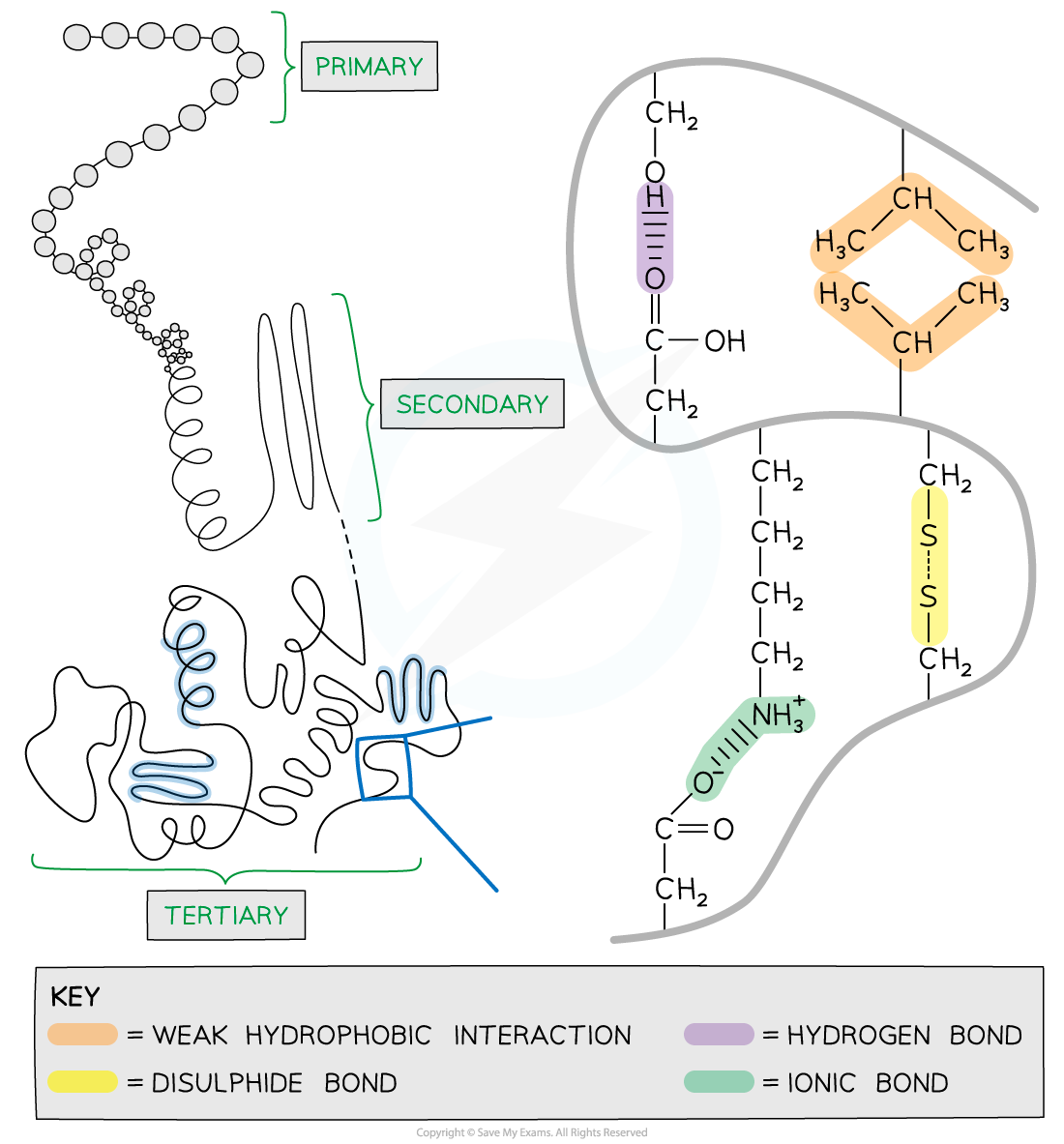
Further conformational change of the secondary structure leads to additional bonds forming between the R groups (side chains)
The additional bonds are:
Hydrogen (these are between R groups)
Disulphide (only occurs between cysteine amino acids)
Ionic (occurs between charged R groups)
Weak hydrophobic interactions (between non-polar R groups)
This structure is common in globular proteins
The structure of tertiary proteins
Type of Bond | Protein Structure | ||
|---|---|---|---|
Primary | Secondary | Tertiary | |
Peptide | ✓ | ✓ | ✓ |
Hydrogen | x | ✓ between NH2 and COOH groups | ✓ between NH2, COOH and R groups |
Disulfide | x | x | ✓ |
Ionic | x | x | ✓ |
Hydrophobic interactions | x | x | ✓ |
Examiner Tips and Tricks
You should be able to draw the peptide formed by the joining of up to three amino acids.
Protein Hydrolysis
Hydrolysis of proteins is the reverse reaction of condensation in which the peptide link is broken and water added, hence the term hydrolysis
During hydrolysis reactions polypeptides are broken down to amino acids when the addition of water breaks the peptide bonds
The hydrolysis reaction can be carried out by chemical means or using enzymes
Concentrated hydrochloric acid is the reagent used and the mixture is boiled for many hours as the reaction is slow
With enzymes, the reaction occurs at room temperature
Hydrolysis of proteins
Identification of Amino Acids
After hydrolysis, the amino acid components from polypeptides can be identified by using the technique of thin layer chromatography (TLC)
Although the amino acids have the same basic structure the R group changes the overall polarity of the molecule so the amino acids will rise up the TLC at different rates
Since amino acids are colourless the TLC plate has to either be sprayed with a locating agent such as ninhydrin which stains the amino acids, or the plate must be illuminated under a UV light
A TLC plate can be used to calculate Rf values for compounds
These values can be used alongside other analytical data to deduce composition of mixtures
The Rf value (retention factor) can be determined and use to identify a specific amino acid

Two dimensional TLC
Sometimes amino acids have very similar values in the same solvent, so a further technique of two dimensional TLC can be used
In this technique the same plate is run through two different solvents
A square TLC plate is used and run through the first solvent, then the plate is turned through 90o and run through the second solvent
Two Rf values are determined allowing greater confidence in identifying the amino acids


You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?

