Addition Reactions of Alkenes (Oxford AQA International A Level (IAL) Chemistry): Revision Note
Exam code: 9622
Electrophilic Addition Mechanism
The presence of a carbon-carbon double bond makes alkenes very reactive compared to alkanes
The C-C double bond is an area of high electron density making it susceptible to attack by electrophiles
Alkenes therefore typically undergo electrophilic addition reactions
Electrophilic addition is the addition of an electrophile to a double bond
The C-C double bond is broken, and a new single bond is formed from each of the two carbon atoms
Reaction with HBr
A molecule of hydrogen bromide (HBr) is polar as the hydrogen and bromine atoms have different electronegativities
The bromine atom has a stronger pull on the electrons in the H-Br bond
As a result of this, the Br atom has a partial negative and the H atom has a partial positive charge
The polarity of a HBr molecule

In electrophilic addition:
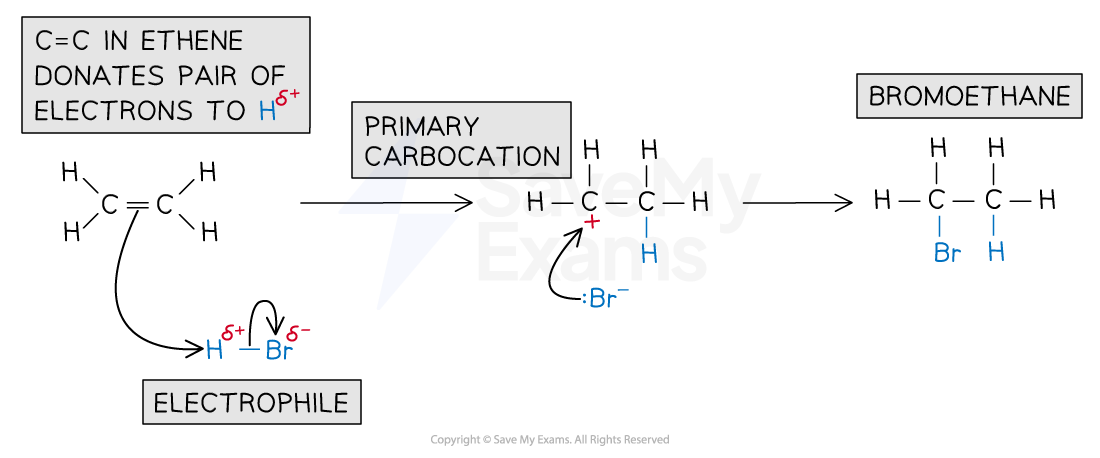
The partially positive (δ+) hydrogen atom acts as an electrophile
It is attracted to the high electron density of the C=C double bond in the alkene and accepts a pair of electrons
The H-Br bond breaks heterolytically, forming a Br- ion
A highly reactive carbocation intermediate is formed which reacts with the bromide ion, Br-
The reaction of ethene with HBr forms bromoethane
Electrophilic addition of HBr mechanism
Reaction with H2SO4
Concentrated sulfuric acid adds across the double bond
The hydrogen atom in sulfuric acid has a partial positive charge so a sulfuric acid molecule acts as electrophile
The polarity of a H2SO4 molecule

In electrophilic addition:
The partially positive (δ+) hydrogen atom acts as an electrophile
It is attracted to the high electron density of the C=C double bond in the alkene and accepts a pair of electrons
The H-O bond breaks heterolytically, forming a hydrogensulfate ion, HSO4-
A highly reactive carbocation intermediate is formed which reacts with the HSO4-

The product formed reacts with water to form an alcohol and sulfuric acid
Essentially, water adds across the double bond with sulfuric acid acting as a catalyst
Reaction with Br2
Bromine (Br2) is a non-polar molecule as both atoms have similar electronegativities and equally share the electrons in the covalent bond
However, when a bromine molecule gets close to the double bond of an alkene, the high electron density in the double bond repels the electron pair in Br-Br away from the closest Br atom
As a result of this, the Br atom closest to the double bond has a partial positive charge (δ+) and the further Br atom has a partial negative charge (δ-)
The polarity of a Br2 molecule

In an addition reaction:
The closest Br atom acts as an electrophile and accepts a pair of electrons from the C=C bond in the alkene
The Br-Br bond breaks heterolytically, forming a Br- ion
This results in the formation of a highly reactive carbocation intermediate which reacts with the Br- (nucleophile)
The reaction of ethene with Br2 forms 1,2-dibromoethane
Electrophilic addition of Br2 mechanism

Testing for unsaturation
The reaction of an alkene with bromine is used to test for the presence of a carbon-carbon double bond
Bromine water is an orange / brown solution
The unknown compound is shaken with the bromine water
If the compound is unsaturated, an addition reaction will take place and the coloured solution will decolourise
The unsaturation test

Examiner Tips and Tricks
Do not state that bromine / bromine water is red as this is normally marked as reject / do not accept on a mark scheme, which means you will lose a mark.
Addition Reactions of Unsymmetrical Alkenes
Carbocations are reaction intermediates that contain positively charged carbon atoms with only three covalent bonds instead of four
There are three types of carbocations:
Primary
Secondary
Tertiary
The inductive effect
The alkyl groups attached to the positively charged carbon atoms are ‘electron donating groups’
This is also known as the positive inductive effect of alkyl groups
The inductive effect is illustrated by the use of arrowheads on the bonds to show the alkyl groups pushing electrons towards the positively charged carbon
This causes the carbocation to become less positively charged
As a result of this, the charge is spread around the carbocation which makes it energetically more stable
Primary carbocations are the least stable as they only have one electron-donating alkyl group to stabilise the carbocation
Secondary carbocations are more stable as they have two electron-donating alkyl group to stabilise the carbocation
Tertiary carbocations are the most stable as they have three electron-donating alkyl groups to stabilise the carbocation
Due to the positive charge on the carbon atom, carbocations are electrophiles
Primary, secondary and tertiary carbocations

Markovnikov’s rule
Markovnikov’s rule predicts the outcome of electrophilic addition reactions and states that:
In the electrophilic addition reaction of a hydrogen halide (HX) to an alkene, the product has the halogen bonded to the most substituted carbon atom
In the electrophilic addition reaction of a halogen to an alkene, each halogen atom bonds to one of the C=C carbons
In the electrophilic addition reaction of an interhalogen (e.g. Br-Cl) to an alkene, the most electronegative halogen ends up bonded to the most substituted carbon atom
Markovnikov addition applies to electrophilic addition reactions with unsymmetrical alkenes such as propene and but-1-ene
Markovnikov addition favours the formation of the major product
Anti-Markovnikov addition favours the formation of the minor product
In electrophilic addition reactions, an electrophile reacts with the double bond of alkenes (as previously discussed)
The mechanism for electrophilic addition reactions with unsymmetrical alkenes is slightly different
The example of propene with HBr is shown below
Step 1 in the electrophilic addition mechanism

The electrophile can attach in two possible ways:
Breaking the C=C bond and attaching to the least substituted carbon
This will give the most stable carbocation as an intermediate that will form the major product
Breaking the C=C bond and attaching to the most substituted carbon
This will give the least stable carbocation as an intermediate that will form the minor product
Relative stabilities of primary and secondary carbocations

The nucleophile will bond to the positive carbon atom of the carbocation
The more stable carbocation produces the major product
The less stable carbocation produces the minor product
Formation of major and minor products

Propene + HBr mechanism
The mechanism for the electrophilic addition of hydrogen bromide to propene, showing the formation of the major and minor products can be shown as:

Examiner Tips and Tricks
The stability of the carbocation intermediate is as follows:
tertiary > secondary > primary
When more than one carbocation can be formed, the major product is formed from the most stable carbocation

Unlock more, it's free!
Did this page help you?