Reduction of Carbonyls (Oxford AQA International A Level (IAL) Chemistry): Revision Note
Exam code: 9622
Reduction of Carbonyls
Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohols
Possibly the most common reducing agent for this is sodium tetrahydridoborate, NaBH4
You may also see this named as sodium borohydride in some sources
In an aqueous solution, NaBH4 generates the hydride ion nucleophile, :H-
The hydride ion will reduce a carbonyl group in an aldehyde or a ketone, but is not strong enough to reduce a C=C double bond
This is because it is attracted to the C in the C=O bond, but is repelled by the high electron density of the C=C bond
When this reaction takes place, it is an example of a nucleophilic addition reaction
Aldehyde to a primary alcohol

Ketone to a secondary alcohol

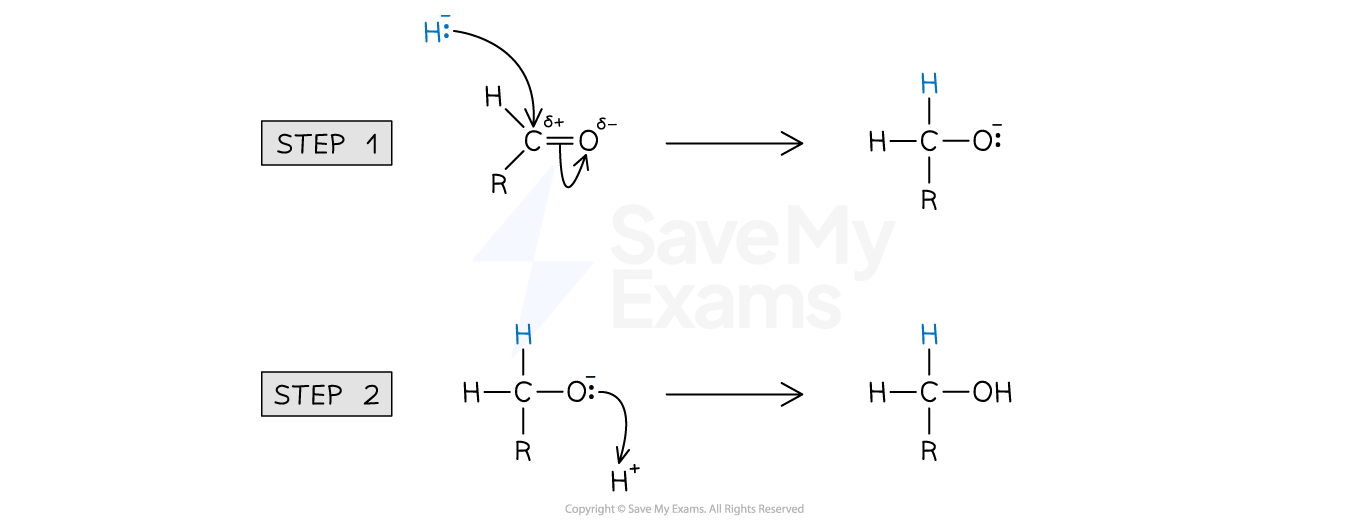
Nucleophilic addition mechanism
The nucleophilic addition mechanism involves two steps
Step 1:
Nucleophilic attack: The lone pair of electrons from the nucleophile attacks the partially positive carbon atom on the carbonyl group
Step 2:
New O-H bond forms: The lone pair of electrons from the negative oxygen atom attracts and forms a bond with H+ ion from the solvent
General mechanism for nucleophilic addition

Unlock more, it's free!
Did this page help you?