Classification (Oxford AQA International A Level (IAL) Chemistry) : Revision Note
The Periodic Table: Structure & Classification
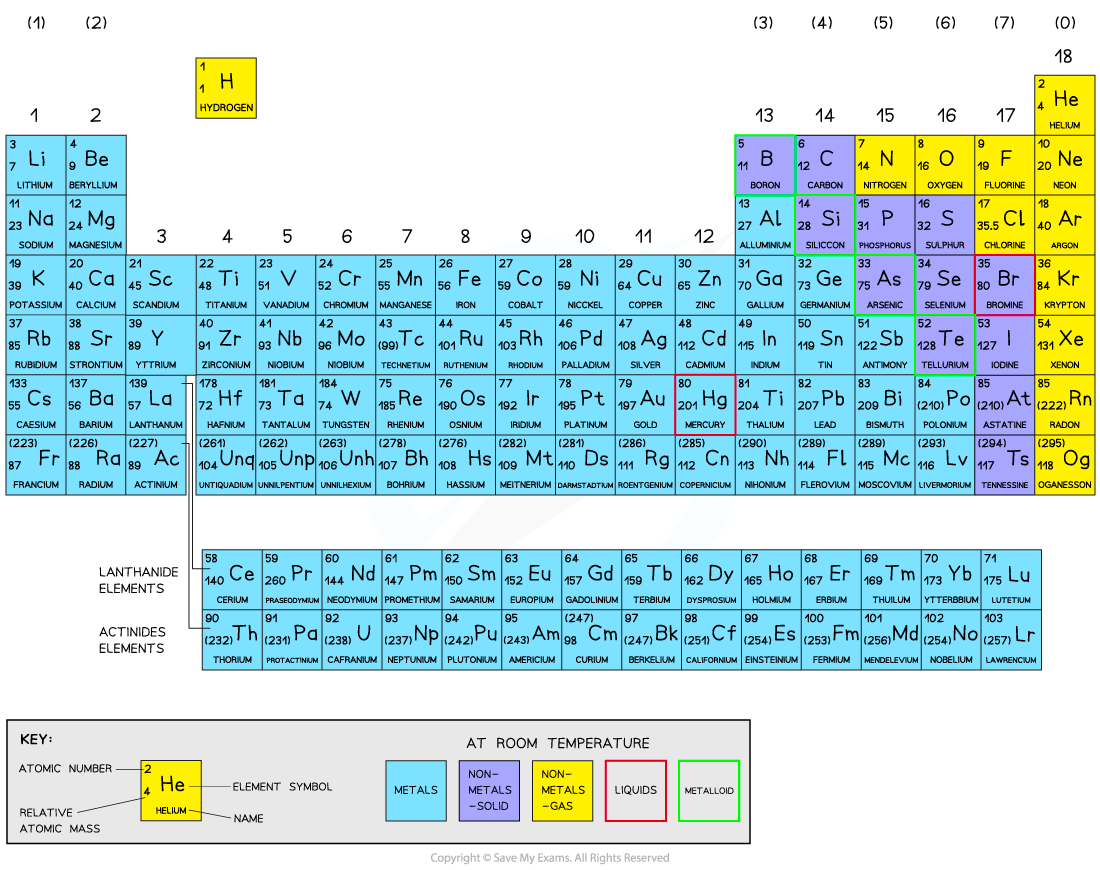
The Periodic Table is a list of all known elements arranged in order of increasing atomic number (proton number)
Elements are arranged in rows called periods
Atoms with the same number of shells are in the same period
Elements are arranged in columns called groups
Atoms with similar electronic configurations in the outer shell are placed in groups
The Periodic Table
All elements belong to one of four main blocks:
s-block
p-block
d-block
f-block
The block an element is in indicates which sub-shell its outer electron is found in
E.g. Magnesium has the electronic configuration 1s22s22p63s3 o is located in p- block
The blocks in the Periodic Table

Elements in the same group show similar chemical properties
As atomic number increases, the properties of the elements show trends which repeat themselves in each period of the periodic table
These trends are known as periodic trends and the study of these trends in known as periodicity

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?

