Activation Energy (Edexcel International A Level (IAL) Chemistry): Revision Note
Exam code: YCH11
Core Practical 10: Finding the Activation Energy of a Reaction
In this practical the activation energy for the reaction between bromide ions and bromate(V) ions will be determined
BrO3- + 5Br- + 6H+ → 3Br2 + 3H2O
C6H5OH + 3Br2 → C6H2Br3OH + 3HBr
The bromine produced in the first reaction reacts with the phenol
When the phenol is ‘used up’, the bromine is no longer removed
The bromine then bleaches the indicator at the ‘end of the reaction’
Common indicators for this reaction are methyl red and methyl orange
Steps in procedure
Pipette 10.0 cm3 of phenol solution and 10.0 cm3 of a bromide / bromate solution into one boiling tube
Add four drops of methyl red indicator to the mixture
Pipette 5.0 cm3 of sulfuric acid solution into a separate boiling tube
Use a kettle and a beaker to prepare a water bath with a temperature of 37 °C (±1 °C) and stand the two boiling tubes in the water bath
When the contents of the boiling tubes have reached the water temperature, mix the contents of the two tubes by pouring rapidly from one tube into the other and then pouring the mixture back into the empty test tube
Start the stop clock at the same time
Leave the boiling tube containing the reaction mixture in the water and time until the methyl red indicator disappears
Record the results
Repeat the whole experiment at different temperatures
Use ice to achieve the lowest temperature if required
Sample Data

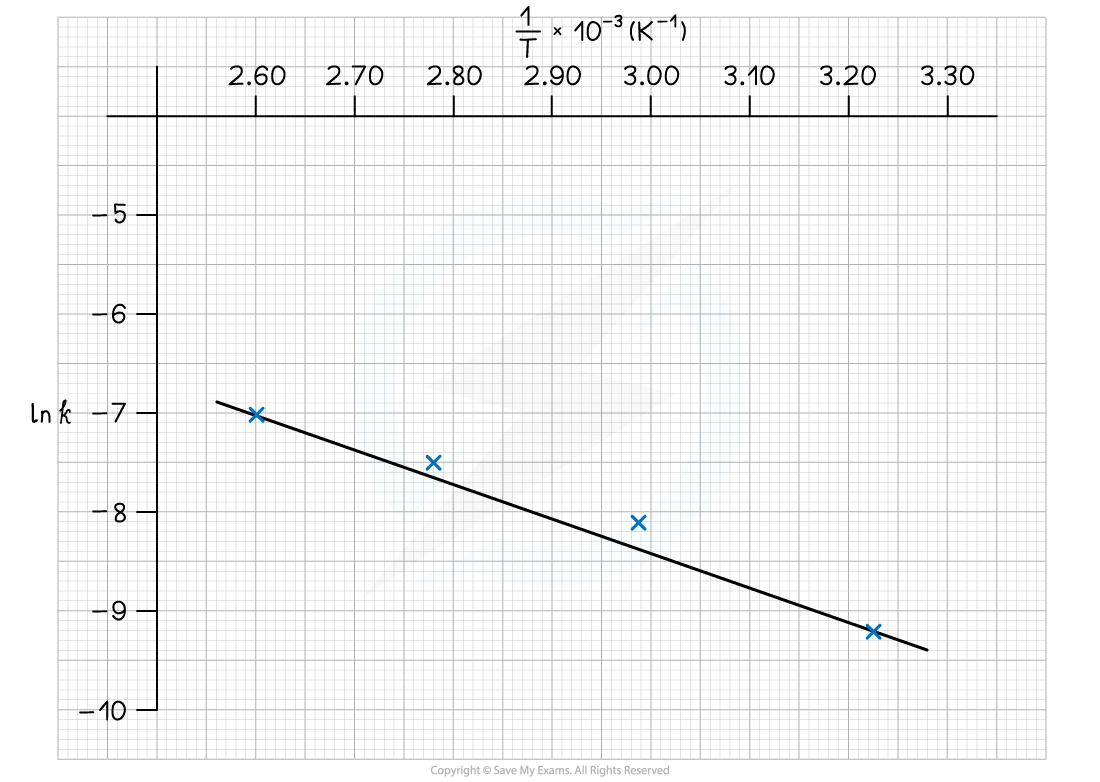
Graph plotted from data





You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?