Haemoglobin & Ligand Exchange (Edexcel International A Level (IAL) Chemistry): Revision Note
Exam code: YCH11
Haemoglobin & Ligand Exchange
Haemoglobin is one of nature's complexes using a transition metal ion
The haem molecule is a complex with iron(II) at its centre
The haemoglobin complex contains a multidentate ligand made up of four haem groups
These consist of mostly carbon and hydrogen atoms
Each haem group has a nitrogen atom forming a dative covalent bond to the Fe2+ ion in a square planar complex
There is a fifth dative bond from the protein (globin) to the Fe2+ ion
Oxygen atoms form a dative covalent bond with the iron(II) which enables oxygen molecules to be transported around the body in the blood

The haem molecule with iron(II) at its centre
Oxygen molecules are not very good ligands and bond weakly to the iron(II)
The weak bonds allows them to break off easily and be transported into cells
Examiner Tips and Tricks
You do not need to be familiar with the structure of the haem group
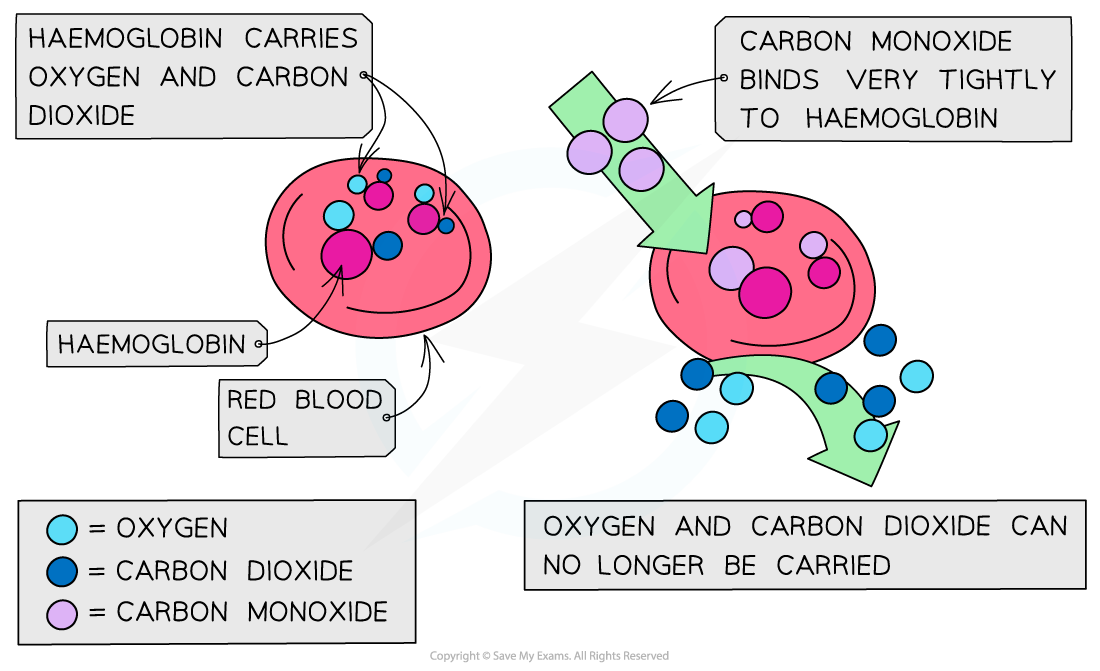
Carbon monoxide is toxic because it is a better ligand than oxygen and binds strongly to the iron(II) preventing oxygen from being carried to the cells
The reason for the strength of the carbon monoxide binding more strongly to haemoglobin is that it forms stronger dative covalent bonds, although it can be displaced from heamoglobin
If oxygen attached to the haemoglobin (oxyhaemoglobin) is replaced by carbon monoxide (carboxyhaemoglobin), a darker red colour is produced in the haem complex
This is a sign of carbon monoxide poisoning
The condition anaemia occurs when a person does not have enough haemoglobin in their blood due to a loss of blood or deficiency in iron
Deficiency in iron can be restored by taking iron sulfate tables in the diet

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?