Preparation of Carboxylic Acids (Edexcel International A Level (IAL) Chemistry): Revision Note
Exam code: YCH11
Carboxylic Acids - Preparation
Carboxylic acids are compounds with a -COOH functional group
They can be prepared by a series of different reactions
Oxidation of primary alcohols & aldehydes
Carboxylic acids can be formed from the oxidation of primary alcohols and aldehydes by either acidified K2Cr2O7 or acidified KMnO4 and reflux
The oxidising agents themselves get reduced causing the solutions to change colour
In K2Cr2O7 the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
In KMnO4 the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
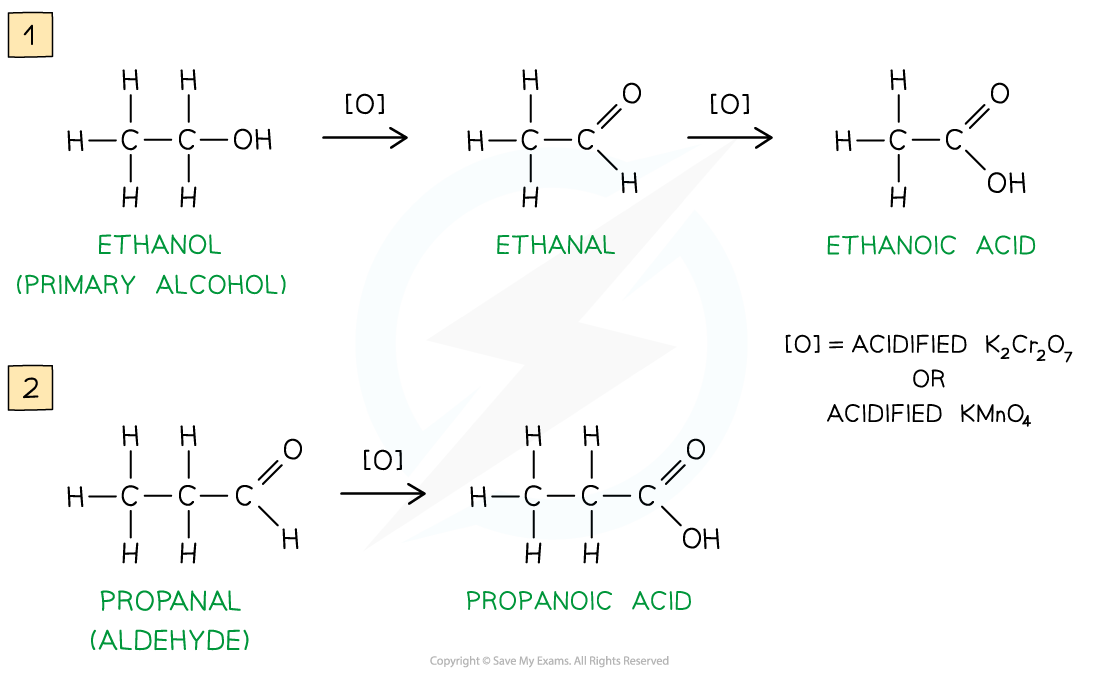
Oxidation of primary alcohols (1) and aldehydes (2) gives carboxylic acids
Hydrolysis of nitriles
Carboxylic acids can also be prepared from the hydrolysis of nitriles using either dilute acid or dilute alkali followed by acidification
Hydrolysis by dilute acid results in the formation of a carboxylic acid and ammonium salt
Hydrolysis by dilute alkali results in the formation of a sodium carboxylate salt and ammonia; Acidification is required to change the carboxylate ion into a carboxylic acid
The -CN group at the end of the hydrocarbon chain is converted to a -COOH group

Hydrolysis of nitriles by either dilute acid (1) or dilute alkali and acidification (2) will form a carboxylic acid

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?