Reaction Feasibility (Edexcel International A Level (IAL) Chemistry): Revision Note
Exam code: YCH11
Reaction Feasibility
Summary
For ΔStotal to be positive and therefore the reaction feasible:
Both ΔSsystem and ΔSsurroundings are positive
ΔSsurroundings is positive and ΔSsystem is negative, but ΔSsurroundings > ΔSsystem
ΔSsurroundings is negative and ΔSsystem is positive, but ΔSsurroundings < ΔSsystem
Feasibility
Generally, entropy will increase in the order:
solid < liquid < gas
Therefore we can determine the change in entropy and the feasibility by considering the change in state of reactants to products
Example 1: the reaction between magnesium and oxygen at 293 K is feasible
Mg (s) + O2 (g) → MgO (s)
This reaction produces a solid from a solid and a gas. Therefore entropy of the system is negative (ΔSsystem < ΔSsurroundings)
But since the entropy of the surroundings is very large as this reaction is very exothermic
This outweighs the entropy of the system, so the total entropy is positive therefore the reaction is spontaneous
Example 2: the reaction between ethanoic acid and ammonium carbonate
2CH3COOH (aq) + (NH4)2CO3 (s) → 2CH3COONH4 (aq) + H2O (l) + CO2 (g)
This reaction is endothermic, therefore ΔSsurroundings is negative
However, a gas is produced from a solid and liquid, so ΔSsystem is positive
ΔSsystem > ΔSsurroundings and the reaction is spontaneous
Gibbs free energy
We can also use Gibbs free energy to work out if a reaction is feasible or not (this is not required as a part of the course)
The feasibility of a reaction is determined by two factors
The enthalpy and entropy change
The two factors come together in a fundamental thermodynamic concept called the Gibbs free energy (G)
The Gibbs equation is:
ΔGꝋ = ΔHreactionꝋ – TΔSsystemꝋ
The units of ΔGꝋ are in kJ mol–1
The units of ΔHreactionꝋare in kJ mol–1
The units of T are in K
The units of ΔSsystemꝋ are in J K-1 mol–1(and must therefore be converted to kJ K–1 mol–1by dividing by 1000)
For a reaction to be feasible, ΔGꝋ must be equal or less than zero
Temperature & feasibility
We can look at the the values for ΔH and ΔS to determine whether the reaction is spontaneous / feasible at a given temperature (T)
The Gibbs equation can explain what will affect the spontaneity / feasibility of a reaction for exothermic and endothermic reactions
Exothermic reactions
In exothermic reactions, ΔHreactionꝋ is negative
If the ΔSsystemꝋ is positive:
Both the first and second term will be negative
Resulting in a negative ΔGꝋ so the reaction is feasible
Therefore, regardless of the temperature, an exothermic reaction with a positive ΔSsystemꝋ will always be feasible

If the ΔSsystemꝋ is negative:
The first term is negative and the second term is positive
At very high temperatures, the –TΔSsystemꝋ will be very large and positive and will overcome ΔHreactionꝋ
Therefore, at high temperatures ΔGꝋ is positive and the reaction is not feasible
Since the relative size of an entropy change is much smaller than an enthalpy change, it is unlikely that TΔS > ΔH as temperature increases
These reactions are therefore usually spontaneous at normal conditions
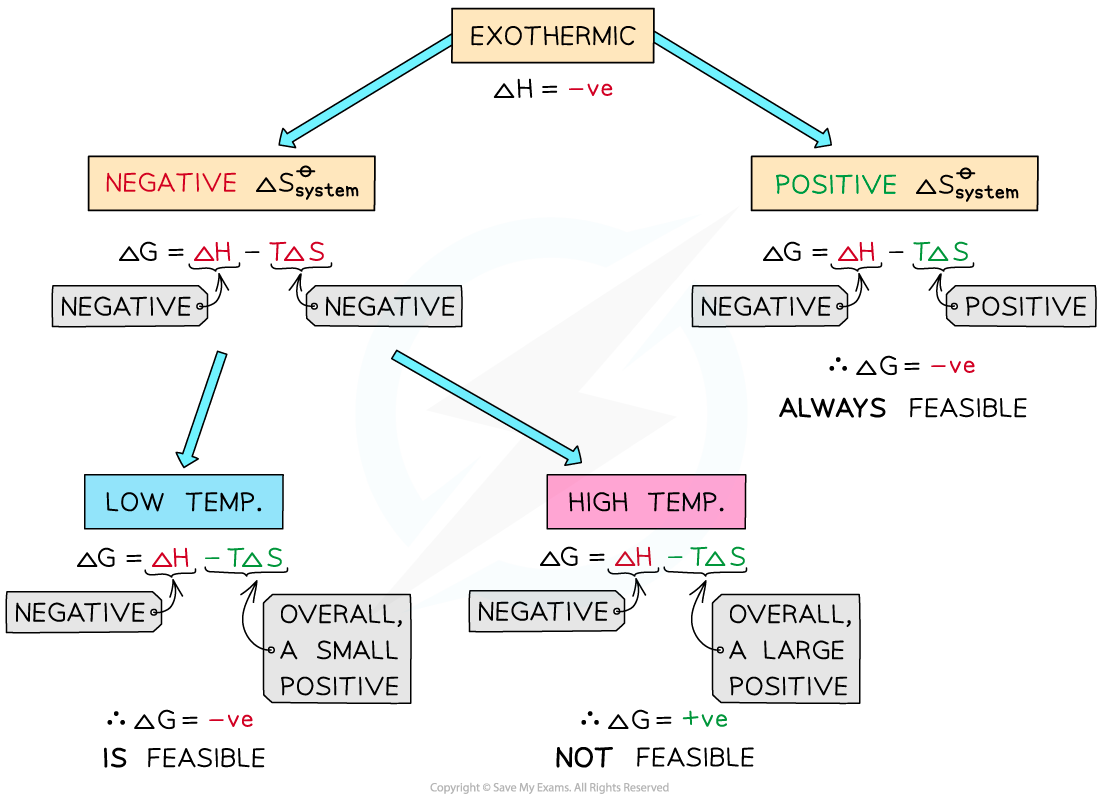
The diagram shows under which conditions exothermic reactions are feasible
Endothermic reactions
In endothermic reactions, ΔHreactionꝋ is positive
If the ΔSsystemꝋ is negative:
Both the first and second term will be positive
Resulting in a positive ΔGꝋ so the reaction is not feasible
Therefore, regardless of the temperature, endothermic with a negative ΔSsystemꝋ will never be feasible
If the ΔSsystemꝋ is positive:
The first term is positive and the second term is negative
At low temperatures, the –TΔSsystemꝋ will be small and negative and will not overcome the larger ΔHreactionꝋ
Therefore, at low temperatures ΔGꝋ is positive and the reaction is not feasible
The reaction is more feasible at high temperatures as the second term will become negative enough to overcome the ΔHreactionꝋ resulting in a negative ΔGꝋ
This tells us that for certain reactions which are not feasible at room temperature, they can become feasible at higher temperatures
An example of this is found in metal extractions, such as the extraction if iron in the blast furnace, which will be unsuccessful at low temperatures but can occur at higher temperatures (~1500 oC in the case of iron)
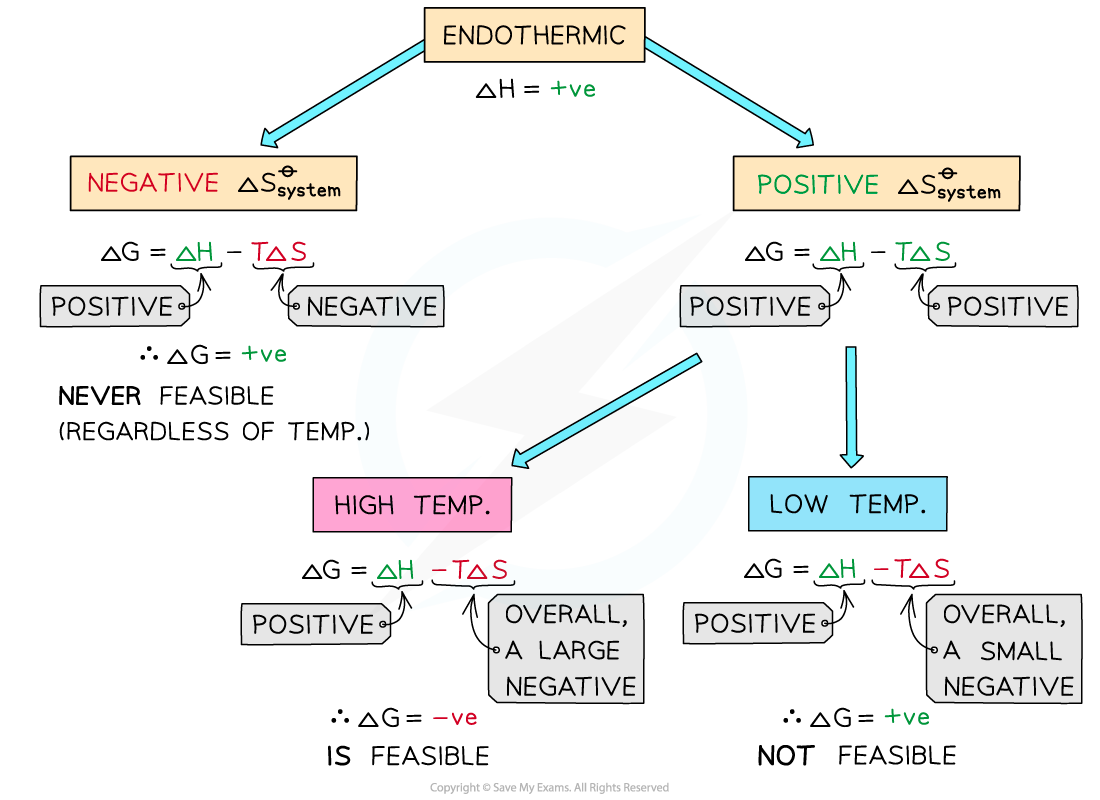
The diagram shows under which conditions endothermic reactions are feasible
Summary of factors affecting Gibbs free energy

Worked Example
The reaction between aluminium oxide and carbon is not feasible at room temperature.
Al2O3 + 3C(s) → 2Al(s) + 3CO2 (g)
Given that ΔH = +1336 kJmol-1 and ΔS= +581 JK-1mol-1 , calculate the temperature at which the reaction becomes feasible.
Answer
As both ΔH and ΔS are positive, ΔG will become negative if TΔS > ΔH.
The temperature at which this reaction becomes spontaneous can be calculated. This will be when ΔG = 0.
If ΔG = 0, then T = ΔH / ΔS*
T =
T = 2299 K
*Don't forget to convert this into kJ
Thermodynamic & Kinetic Stability
Thermodynamic Stability
Refers to whether a reaction is feasible under given conditions
A reaction is thermodynamically feasible if the Gibbs free energy change (ΔG) is negative
ΔG = ΔH−TΔS
This means:
Reactions are more likely to be feasible if enthalpy change (ΔH) is negative (exothermic)
Or if entropy change (ΔS) is positive (increase in disorder)
Thermodynamic feasibility does not necessarily mean the reaction will happen quickly
Kinetic Stability
Refers to how fast a reaction occurs (i.e. the rate of reaction)
Depends on the activation energy (Eₐ):
Reactions with a high Eₐ are kinetically stable and may not occur at room temperature
Even if a reaction is thermodynamically feasible, it might not happen without enough energy to overcome the activation barrier
Examples
Combustion of methane, CH4
Thermodynamically feasible but kinetically stable
Methane (CH4) will not spontaneously form CO2 and H2O unless ignited (Eₐ is high)
Decomposition of hydrogen peroxide at 298 K
This reaction is thermodynamically feasible:
H2O2 (l) → H2O (l) + ½O2 (g)
The activation energy, Ea, is high, so a catalyst must be used (MnO2)
If the reaction was left for long enough, the hydrogen peroxide would eventually decompose, however the addition of the MnO2 allows the reaction to take place via an alternative route with a lower Ea

Unlock more, it's free!
Did this page help you?