Activation Energy & Arrhenius (Edexcel International A Level (IAL) Chemistry): Revision Note
Exam code: YCH11
Activation Energy & Arrhenius
The rate equation shows how each of the reactants in a reaction effects the rate of the reaction and it includes the rate constant, k
However, k only remains constant if the concentration of the reactants is the only factor which is changed
If the temperature is changed or a catalyst is used or changed, then the rate constant, k, changes
At higher temperatures, a greater proportion of molecules have energy greater than than the activation energy
Since the rate constant and rate of reaction are directly proportional to the fraction of molecules with energy equal or greater than the activation energy, then at higher temperatures:
The rate of reaction increases
The rate constant increases
The relationship between the rate constant, the temperature and also the activation energy is given by the Arrhenius equation:

Ea and A are constants that are characteristic of a specific reaction
A does vary slightly with temperature but it can still be considered a constant
R is a fundamental physical constant for all reactions
k and T are the only variables in the Arrhenius equation
The Arrhenius equation is used to describe reactions that involve gases, reactions occurring in solution or reactions that occur on the surface of a catalyst
Finding the Activation Energy
Very often, the Arrhenius equation is used to calculate the activation energy of a reaction
A question will either give sufficient information for the Arrhenius equation to be used or a graph can be plotted and the calculation done from the plot
Using the Arrhenius Equation
The Arrhenius equation is easier to use if you take natural logarithms of each side of the equation, which results in the following equation:

The Arrhenius Equation can be used to show the effect that a change in temperature has on the rate constant, k, and thus on the overall rate of the reaction
An increase in temperature (higher value of T) gives a greater value of ln k and therefore a higher value of k
Since the rate of the reaction depends on the rate constant, k, an increase in k also means an increased rate of reaction
The equation can also be used to show the effect of increasing the activation energy on the value of the rate constant, k
An increase in the activation energy, Ea, means that the proportion of molecules which possess at least the activation energy is less
This means that the rate of the reaction, and therefore the value of k, will decrease
The values of k and T for a reaction can be determined experimentally
These values of k and T can then be used to calculate the activation energy for a reaction
This is the most common type of calculation you will be asked to do on this topic
Worked Example
Calculate the activation energy of a reaction which takes place at 400 K, where the rate constant of the reaction is 6.25 x 10-4 s-1.
A = 4.6 x 1013 and R = 8.31 J mol-1 K-1.
Answer

Using an Arrhenius plot:
A graph of ln k against 1/T can be plotted, and then used to calculate Ea
This gives a line which follows the form y = mx + c
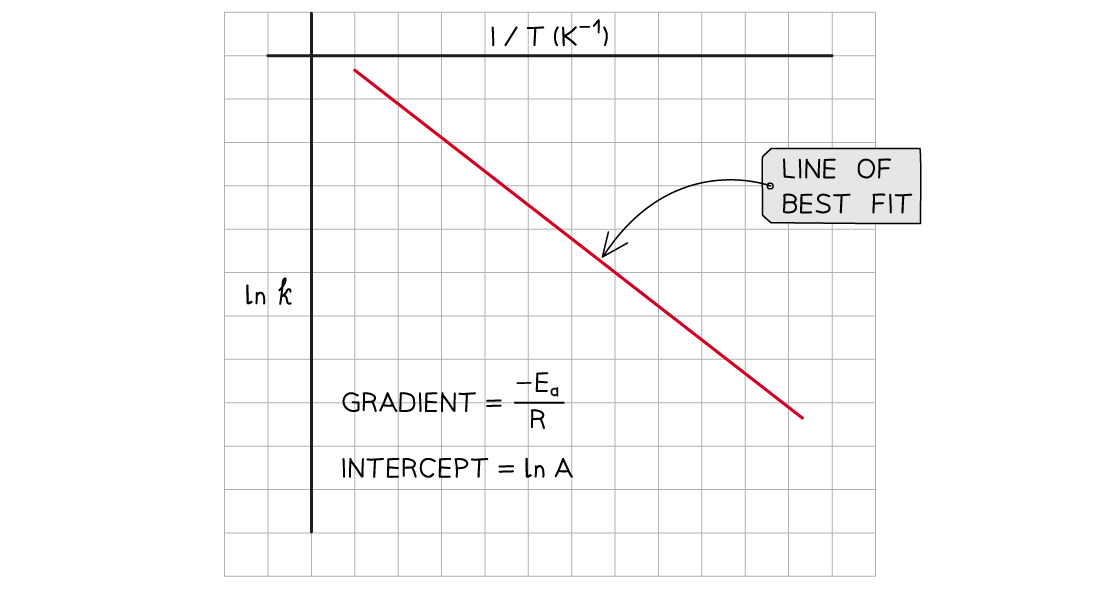
The graph of ln k against 1/T is a straight line with gradient -Ea/R
From the graph, the equation in the form of y = mx + c is as follows:

Worked Example
Complete the following table
Plot a graph of ln k against 1/T
Use this to calculate the activation energy, Ea, and the Arrhenius constant, A, of the reaction.

Answer 1:

Answer 2:
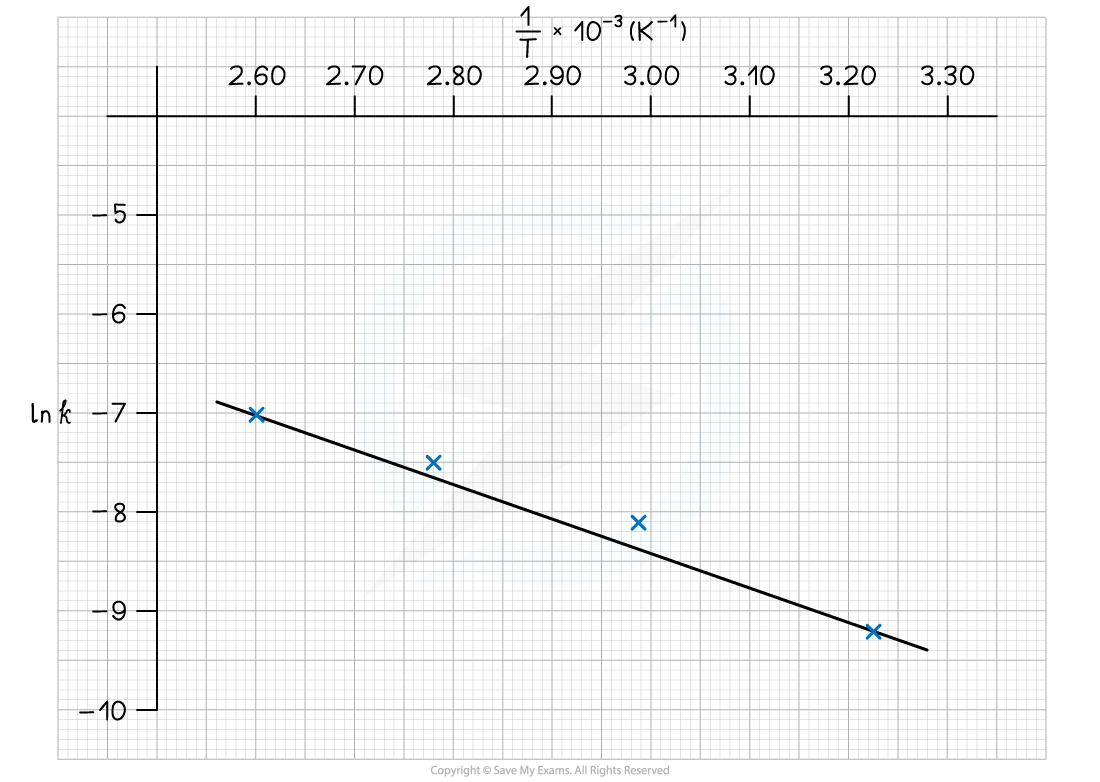
Answer 3:





You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?