Enthalpy of Solution - Calculations
- Questions in this topic typically ask you to calculate the hydration enthalpy of one of the ions, given the lattice enthalpy, enthalpy of solution and hydration enthalpy of the other ion.
- This can be done by constructing an appropriate energy cycle and using Hess's Law to find the unknown energy value
- The energy cycle above shows that there are two routes to go from the gaseous ions to the ions in an aqueous solution:
-
- Route 1: going from gaseous ions → ionic solid → ions in aqueous solution (this is the indirect route)
- Route 2: going from gaseous ions → ions in aqueous solution (this is the direct route)
-
- According to Hess’s law, the enthalpy change for both routes is the same, such that:
ΔhydHꝋ = ΔlattHꝋ + ΔsolHꝋ
- Each ion will have its own enthalpy change of hydration, ΔHhydꝋ, which will need to be taken into account during calculations
- The total ΔhydHꝋ is found by adding the ΔhydHꝋ values of both anions and cations together
Worked example
Constructing an energy cycle for KCl
Calculate the enthalpy of hydration of the chloride ion given the following data:
ΔlattHꝋ [KCl] = -711 kJ mol-1
ΔsolHꝋ [KCl] = +26 kJ mol-1
ΔhydHꝋ [K+] = -322 kJ mol-1
Answer
Step 1: Draw the energy cycle and make ΔhydH[Cl-] the subject of the formula:

Step 2: Substitute the values to find ΔhydH[Cl-]
ΔhydH[Cl-] = (-711) + (+26) - (-322) = -363 kJ mol-1
Alternative Diagram
- You can also draw a Born-Haber cycle as an alternative approach to the same problem
Energy level diagram:


Worked example
Constructing an energy cycle and energy level diagram of MgCl2
Construct an energy cycle to calculate the ΔhydHꝋof magnesium ions in magnesium chloride, given the following data:
ΔlattH [MgCl2] = -2592 kJ mol-1
ΔsolH [MgCl2] = -55 kJ mol-1
ΔhydH [Cl-] = -363 kJ mol-1
Answer
Step 1: Draw an energy cycle:
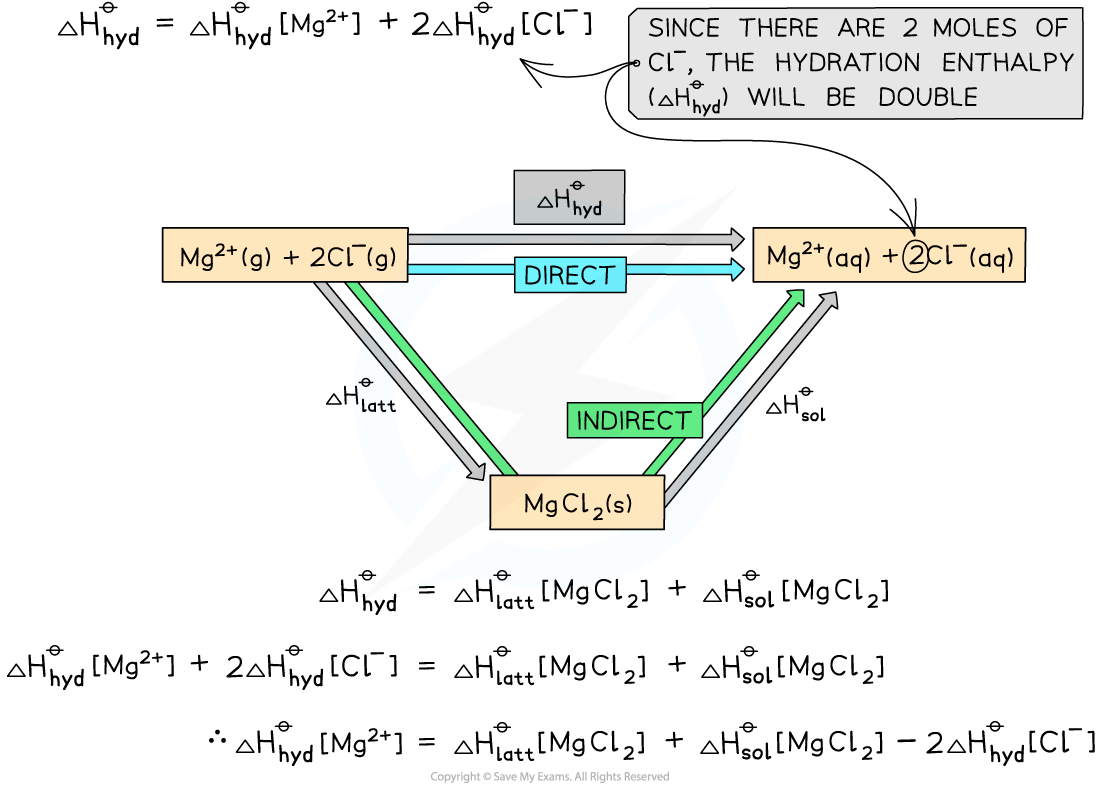
Step 2: Substitute the values to find ΔHhydꝋ [Mg2+]
ΔHhydꝋ[Mg2+] = (-2592) + (-55) - (2 x -363) = -1921 kJ mol-1
Alternative route to find ΔhydHꝋ[Mg2+]
- Here is the same solution using a Born-Haber cycle

Examiner Tip
It doesn't matter whether you use Hess cycles or Born-Haber style cycles to solve these problems as long as the information is correctly labelled and the direction of the arrows matches the definitions.Exam problems in this topic often show diagrams with missing labels which you have to complete and find unknown values.The key to success in energy cycle calculations is not to panic, but have a careful step-by-step approach, show your workings and use brackets to separate mathematical operations from the enthalpy changes.

