Rates of Reaction (Edexcel International A Level (IAL) Chemistry): Revision Note
Exam code: YCH11
Rates of Reaction - Graphs & Calculations
Some reactions take place instantly, but most are much slower and it is possible to measure how long these reactions take to reach a certain stage
As a chemical reaction proceeds, the concentration of the reactants decreases and the concentration of the products increases
The rate of a reaction is the speed at which a chemical reaction takes place and has units mol dm-3 s-1
The rate of a reaction can be calculated by:
Rate of reaction =
Graphically we can represent the rate of reaction as:

Rate of reaction graphs
Worked Example
Iodine and methanoic acid react in aqueous solution.
I2 (aq) + HCOOH (aq) → 2I− (aq) + 2H+ (aq) + CO2 (g)
The rate of reaction can be found by measuring the volume of carbon dioxide produced per unit time and plotting a graph as shown
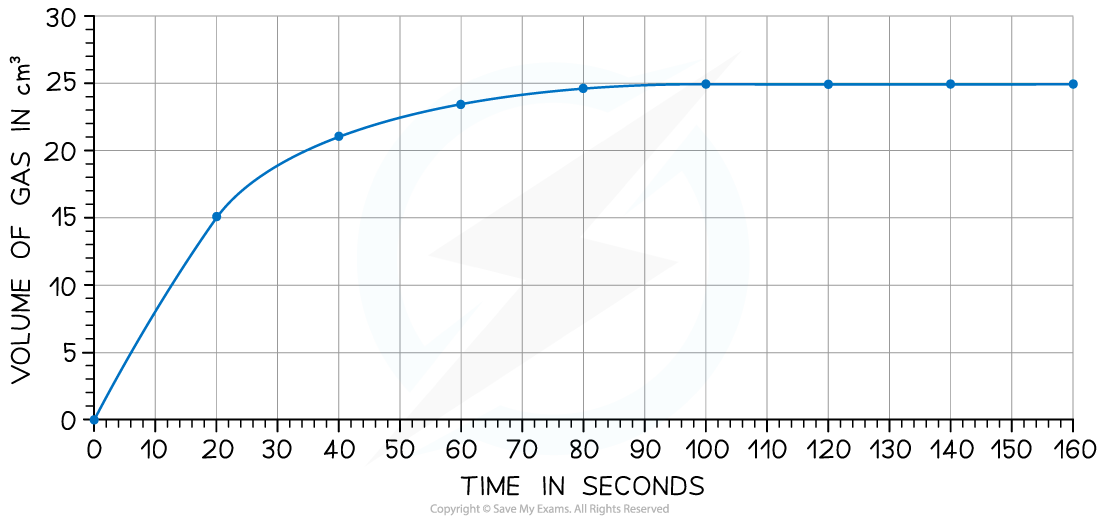
Calculate the rate of reaction at 20 seconds
Answer:
Draw a tangent to the curve at 20 seconds:

Complete the triangle and read off the values of x and y
Determine the gradient of the line using ∆y / ∆x
Rate of reaction = 24 ÷ 40 = 0.60 cm3 s-1
Examiner Tips and Tricks
When drawing the tangent to a curve make the triangle large and try to intersect with gridlines if you can. This minimises errors of precision and reduces the chance you will accidently misread the graph values
To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed
The method used for measuring depends on the substances involved
There are a number of ways to measure a reaction rate in the lab; they all depend on some property that changes during the course of the reaction
That property is taken to be proportional to the concentration of the reactant or product, e.g., colour, mass, volume
Some reaction rates can be measured as the reaction proceeds (this generates more data);
faster reactions can be easier to measure when the reaction is over, by averaging a collected measurement over the course of the reaction
Commonly used techniques are:
mass loss
gas production
Changes in mass
When a gas is produced in a reaction it usually escapes from the reaction vessel, so the mass decreases
This can be used to measure the rate of reaction
For example, the reaction of calcium carbonate with hydrochloric acid produces CO2
The mass is measured every few seconds and change in mass over time is plotted as the CO2 escapes

Measuring changes in mass using a balance
The mass loss provides a measure of the amount of reactant, so the graph is the same as a graph of amount of reactant against time

Mass loss of a product against time
However, one limitation of this method is the gas must be sufficiently dense or the change in mass is too small to measure on a 2 or 3 d.p. balance
So carbon dioxide would be suitable (Mr = 44.0) but hydrogen would not (Mr = 2.0)
Volumes of gases
When a gas is produced in a reaction, it can be trapped and its volume measured over time
This can be used to measure the rate of reaction.
For example, the reaction of magnesium with hydrochloric acid produces hydrogen

Collecting gases experimental set up
An alternative gas collection set up involves collecting a gas through water using an inverted measuring cylinder (as long as the gas is not water soluble)

Alternative gas collection set up
The volume can be measured every few seconds and plotted to show how the volume of gas varies with time
The volume provides a measure of the amount of product, so the graph is a graph of amount of product against time

Graph of gas volume evolved against time
Measuring concentration changes
Measuring concentration changes during a reaction is not easy; the act of taking a sample and analysing it by titration can affect the rate of reaction (unless the reaction is deliberately stopped- this is called quenching).
Often it is more convenient to ‘stop the clock’ when a specific (visible) point in the reaction is reached
For example when a piece of magnesium dissolves completely in hydrochloric acid
Another common rate experiment is the reaction between sodium thiosulfate and hydrochloric acid which slowly produces a yellow precipitate of sulfur that obscures a cross when viewed through the solution:
Na2S2O3 (aq) + 2HCl (aq) → 2NaCl aq) + SO2 (g) + H2O (l) + S(s)

The disappearing cross experiment
The main limitation here is that often it only generates one piece of data for analysis
Worked Example
Using the results shown below, calculate the initial rate of reaction for the reaction using 2.0 mol dm-3 HCl (aq)
Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)

Answer
Step 1: Draw a graph of the results

The gradient can be used to give the rate of reaction, however, the graph has produced a curve
Step 2: Draw a tangent to the curve at time = 0 seconds
Step 3: Calculate the gradient
Gradient
1.05 mol dm-3 s-1
Examiner Tips and Tricks
You should be familiar with the interpretation of graphs of changes in concentration, volume or mass against time and be able to calculate a rate from a tangent to the graph

Unlock more, it's free!
Did this page help you?