The Role of Haemoglobin (Edexcel International A Level Biology): Revision Note
Haemoglobin Structure & Function
Transport of oxygen
The majority of oxygen transported around the body is bound to the protein haemoglobin in red blood cells
Red blood cells are also known as erythrocytes
Each molecule of haemoglobin contains four haem groups, each able to bond with one molecule of oxygen
This means that each molecule of haemoglobin can carry four oxygen molecules, or eight oxygen atoms in total
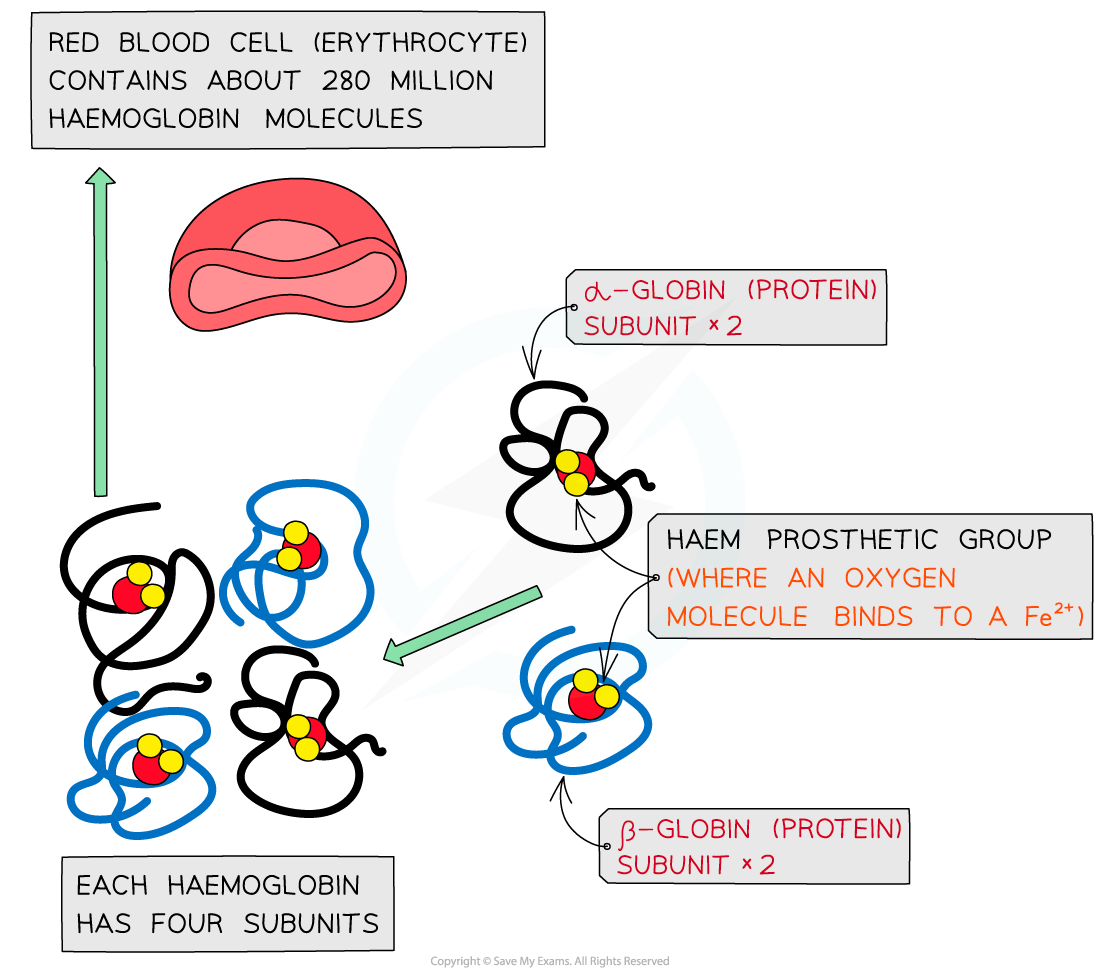
Haemoglobin proteins are made up of four subunits, each of which contains a region called a haem group to which oxygen can bind
When oxygen binds to haemoglobin, oxyhaemoglobin is formed
Oxygen + Haemoglobin Oxyhaemoglobin
4O2 + Hb Hb4O 2
The binding of the first oxygen molecule results in a conformational change in the structure of the haemoglobin molecule, making it easier for each successive oxygen molecule to bind; this is cooperative binding
The reverse of this process happens when oxygen dissociates in the tissues
Carbon dioxide transport
Waste carbon dioxide produced during respiration diffuses from the tissues into the blood
There are three main ways in which carbon dioxide is transported around the body
A very small percentage of carbon dioxide dissolves directly in the blood plasma and is transported in solution
Carbon dioxide can bind to haemoglobin, forming carbaminohaemoglobin
A much larger percentage of carbon dioxide is transported in the form of hydrogen carbonate ions (HCO3-)
Formation of hydrogen carbonate ions
Carbon dioxide diffuses into red blood cells
Inside red blood cells, carbon dioxide combines with water to form H2CO3
CO2 + H2O ⇌ H2CO3
Red blood cells contain the enzyme carbonic anhydrase which catalyses the reaction between carbon dioxide and water
Without carbonic anhydrase this reaction proceeds very slowly
The plasma contains very little carbonic anhydrase hence H2CO3 forms more slowly in plasma than in the cytoplasm of red blood cells
Carbonic acid dissociates readily into H+ and HCO3- ions
H2CO3 ⇌ HCO3– + H+
Hydrogen ions can combine with haemoglobin, forming haemoglobinic acid and preventing the H+ ions from lowering the pH of the red blood cell
Haemoglobin is said to act as a buffer in this situation
The hydrogen carbonate ions diffuse out of the red blood cell into the blood plasma where they are transported in solution

Carbon dioxide can be transported in the form of hydrogen carbonate ions
Association & Dissociation of Haemoglobin
The oxygen dissociation curve
The oxygen dissociation curve shows the rate at which oxygen associates, and also dissociates, with haemoglobin at different partial pressures of oxygen (pO2)
Partial pressure of oxygen refers to the pressure exerted by oxygen within a mixture of gases; it is a measure of oxygen concentration
Haemoglobin is referred to as being saturated when all of its oxygen binding sites are taken up with oxygen; so when it contains four oxygen molecules
The ease with which haemoglobin binds and dissociates with oxygen can be described as its affinity for oxygen
When haemoglobin has a high affinity it binds easily and dissociates slowly
When haemoglobin has a low affinity for oxygen it binds slowly and dissociates easily
In other liquids, such as water, we would expect oxygen to becomes associated with water, or to dissolve, at a constant rate, providing a straight line on a graph, but with haemoglobin oxygen binds at different rates as the pO2 changes; hence the resulting curve
It can be said that haemoglobin's affinity for oxygen changes at different partial pressures of oxygen

Oxygen binds to haemoglobin at different rates as the partial pressure of oxygen changes; the resulting curve is known as the oxygen dissociation curve
Explaining the shape of the curve
The curved shape of the oxygen dissociation curve for haemoglobin can be explained as follows
Due to the shape of the haemoglobin molecule, it is difficult for the first oxygen molecule to bind to haemoglobin; this means that binding of the first oxygen occurs slowly, explaining the relatively shallow curve at the bottom left corner of the graph
After the first oxygen molecule binds to haemoglobin, the haemoglobin protein changes shape, or conformation, making it easier for the next oxygen molecules to bind; this speeds up binding of the remaining oxygen molecules and explains the steeper part of the curve in the middle of the graph
The shape changes of haemoglobin leading to easier oxygen binding is known as cooperative binding
As the haemoglobin molecule approaches saturation it takes longer for the fourth oxygen molecule to bind due to the shortage of remaining binding sites, explaining the levelling off of the curve in the top right corner of the graph
Interpreting the curve
When the curve is read from left to right, it provides information about the rate at which haemoglobin binds to oxygen at different partial pressures of oxygen
At low pO2, in the bottom left corner of the graph, oxygen binds slowly to haemoglobin; this means that haemoglobin cannot pick up oxygen and become saturated as blood passes through the body's oxygen-depleted tissues
Haemoglobin has a low affinity for oxygen at low pO2, so saturation percentage is low
At medium pO2, in the central region of the graph, oxygen binds more easily to haemoglobin and saturation increases quickly; at this point on the graph a small increase in pO2 causes a large increase in haemoglobin saturation
At high pO2, in the top right corner of the graph, oxygen binds easily to haemoglobin; this means that haemoglobin can pick up oxygen and become saturated as blood passes through the lungs
Haemoglobin has a high affinity for oxygen at high pO2, so saturation percentage is high
Note that at this point on the graph increasing the pO2 by a large amount only has a small effect on the percentage saturation of haemoglobin; this is because most oxygen binding sites on haemoglobin are already occupied
When read from right to left, the curve provides information about the rate at which haemoglobin dissociates with oxygen at different partial pressures of oxygen
In the lungs, where pO2 is high, there is very little dissociation of oxygen from haemoglobin
At medium pO2, oxygen dissociates readily from haemoglobin, as shown by the steep region of the curve; this region corresponds with the partial pressures of oxygen present in the respiring tissues of the body, so ready release of oxygen is important for cellular respiration
At this point on the graph a small decrease in pO2 causes a large decrease in percentage saturation of haemoglobin, leading to easy release of plenty of oxygen to the cells
At low pO2 dissociation slows again; there are few oxygen molecules left on the binding sites, and the release of the final oxygen molecule becomes more difficult, in a similar way to the slow binding of the first oxygen molecule
The Bohr effect
Changes in the oxygen dissociation curve as a result of carbon dioxide levels are known as the Bohr effect, or Bohr shift
When the partial pressure of carbon dioxide in the blood is high, haemoglobin’s affinity for oxygen is reduced
This is the case in respiring tissues, where cells are producing carbon dioxide as a waste product of respiration
This occurs because CO2 lowers the pH of the blood
CO2 combines with water to form carbonic acid
Carbonic acid dissociates into hydrogen carbonate ions and hydrogen ions
Hydrogen ions bind to haemoglobin, causing the release of oxygen
This is a helpful change because it means that haemoglobin gives up its oxygen more readily in the respiring tissues where it is needed
On a graph showing the dissociation curve, the curve shifts to the right when CO2 levels increase
This means that at any given partial pressure of oxygen, the percentage saturation of haemoglobin is lower at higher levels of CO2

The dissociation curve shifts to the right as a result of the Bohr effect. This means that at any given partial pressure of oxygen, the percentage saturation of haemoglobin is lower at higher CO2 levels
Foetal haemoglobin
The haemoglobin of a developing foetus has a higher affinity for oxygen than adult haemoglobin
This is vital as it allows a foetus to obtain oxygen from its mother's blood at the placenta
Foetal haemoglobin can bind to oxygen at low pO2
At this low pO2 the mother's haemoglobin is dissociating with oxygen
On a dissociation curve graph, the curve for foetal haemoglobin shifts to the left of that for adult haemoglobin
This means that at any given partial pressure of oxygen, foetal haemoglobin has a higher percentage saturation than adult haemoglobin
After birth, a baby begins to produce adult haemoglobin which gradually replaces foetal haemoglobin
This is important for the easy release of oxygen in the respiring tissues of a more metabolically active individual

Foetal haemoglobin has a higher affinity for oxygen than adult haemoglobin. This means that at any given pO2, foetal haemoglobin will have a higher percentage saturation than adult haemoglobin

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
