Alpha, Beta & Gamma Radiation (Oxford AQA IGCSE Combined Science Double Award): Revision Note
Exam code: 9204
Alpha, Beta & Gamma Radiation
When an unstable nucleus decays, it emits radiation
Substances that emit radiation are said to be radioactive
There are different types of radiation that can be emitted:
Alpha (α) particles
Beta (β-) particles
Gamma (γ) waves
Neutrons
These changes are spontaneous and random in direction
There is an equal probability of any nucleus decaying
It cannot be known which particular nucleus will decay next
It cannot be known at what time a particular nucleus will decay
The rate of decay is unaffected by the surrounding conditions, or chemical and physical processes
It is only possible to estimate the probability of a nucleus decaying in a given time interval
Composition of Alpha, Beta & Gamma Radiation
Alpha particles
The symbol for alpha particles is α
An alpha particle is a helium nucleus
It is made of 2 protons and 2 neutrons
Alpha particles have a charge of +2
This means they can be affected by an electric field
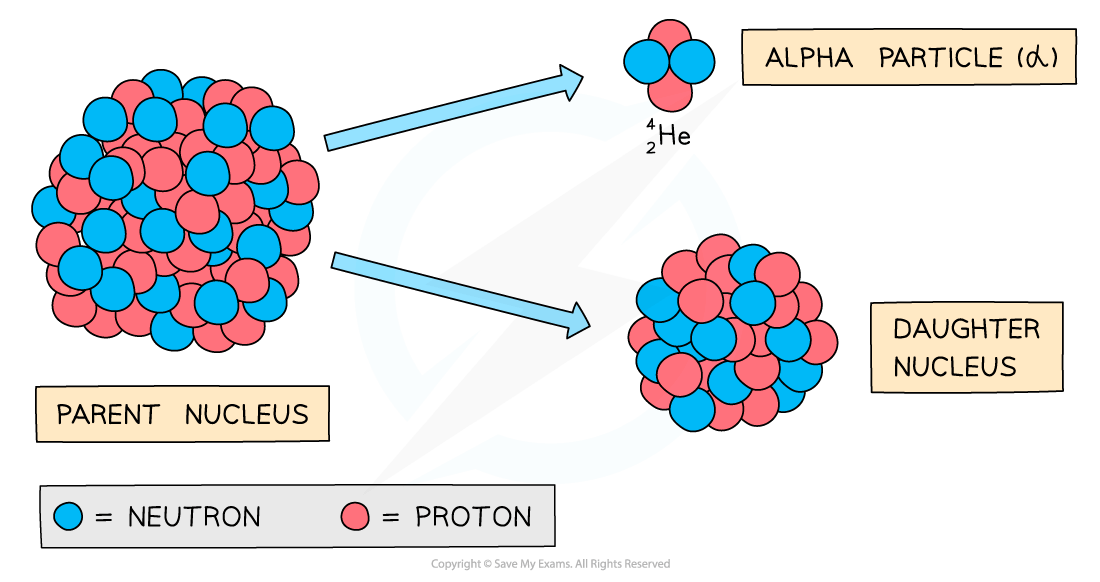
During alpha decay, an alpha particle is emitted from an unstable nucleus
A completely new element is formed in the process because the atomic number changes
An unstable nuclei decaying by alpha radiation
Beta particles
The symbol for beta is β
A beta particle is a high-speed electron
Beta particles have a charge of -1
This means they can be affected by an electric field
During beta decay, a neutron changes into a proton and an electron
The electron is emitted and the proton remains in the nuclei
A completely new element is formed because the atomic number changes
An unstable nuclei decaying by beta radiation

Gamma radiation
The symbol for gamma is γ
Gamma radiation is electromagnetic radiation
They have the highest energy of the different types of electromagnetic radiation
Gamma radiation has no mass or charge
During gamma decay, gamma radiation is emitted from an unstable nucleus
The process that makes the nucleus less energetic but does not change its structure
The atomic number and mass number remain the same
An unstable nuclei decaying by gamma radiation

Neutrons
The symbol for a neutron is n
Neutrons are one of the two particles found in the nucleus of atoms
Neutrons are neutral, they have no charge
Summary of the composition of different types of radiation

Properties of Alpha, Beta & Gamma Radiation
Extension Tier only
The properties of alpha, beta and gamma are given in this table, and then described in more detail below
Different Properties of Nuclear Radiation
Particle | What it is | Charge | Range in Air | Penetration | Ionisation Power |
|---|---|---|---|---|---|
Alpha (α) | 2 protons + 2 neutrons | +2 | Few cm | Stopped by paper | High |
Beta (β) | Electron | -1 | Few 10s of cm | Stopped by few mm of aluminium | Medium |
Gamma (γ) | Electromagnetic radiation | 0 | Infinite | Reduced by few mm of lead | Low |
The trend down the table shows:
The range increases
Penetrating power increases
Ionisation decreases
Penetrating power
Alpha, beta and gamma have different properties
They penetrate materials in different ways
This means they can pass through certain materials and therefore can be stopped by certain materials
Penetrating power of alpha, beta & gamma

Alpha is stopped by paper, whereas beta and gamma pass through it
Beta is stopped by a few millimetres of aluminium
Gamma can pass through aluminium
Gamma rays are only partially stopped by thick lead
Ionising power
All nuclear radiation is capable of ionising atoms that it hits
When an atom is ionised, the number of electrons it has changes
This gives it a non-zero charge
Ionisation of an atom

Alpha radiation is the most ionising form of nuclear radiation
This is because alpha particles have a charge of +2
Gamma radiation is the least ionising form of nuclear radiation
Range in air
The more ionising a form of radiation is, the sooner it will interact with the air it is moving through
Strongly ionising radiation has the shortest range in air
Alpha only travels a few centimetres in air
Beta has a range of a few tens of centimetres
Gamma is not absorbed by air and so has an infinite range, although it does get less intense with distance
Worked Example
A student has an unknown radioactive source. They are trying to work out which of the following types of radiation is being given off:
Alpha particles
Beta particles
Gamma radiation
They measure the count-rate, using a Geiger-Muller tube, when the source is placed behind different materials. Their results are shown in the table below:
Material between Source & Detector | No Material | Paper | 5 mm Aluminium | 5 mm Lead |
|---|---|---|---|---|
Count-rate | 4320 | 4218 | 256 | 34 |
Which type of radiation is being given off by the source?
Answer: Beta particles
The radiation passed through the paper almost unchanged
This means it is not alpha
The aluminium decreased the count-rate significantly
This means it is not gamma (gamma penetrates aluminium)
Therefore, the source must be beta particles

Unlock more, it's free!
Did this page help you?