States of Matter (Oxford AQA IGCSE Combined Science Double Award): Revision Note
Exam code: 9204
States of Matter
All matter is made up of very small particles, or atoms
Kinetic theory is a model that describes the arrangement and movement of particles in a substance
It can be used to explain
The different states of matter e.g. solids, liquids and gases
Physical properties e.g. differences in density
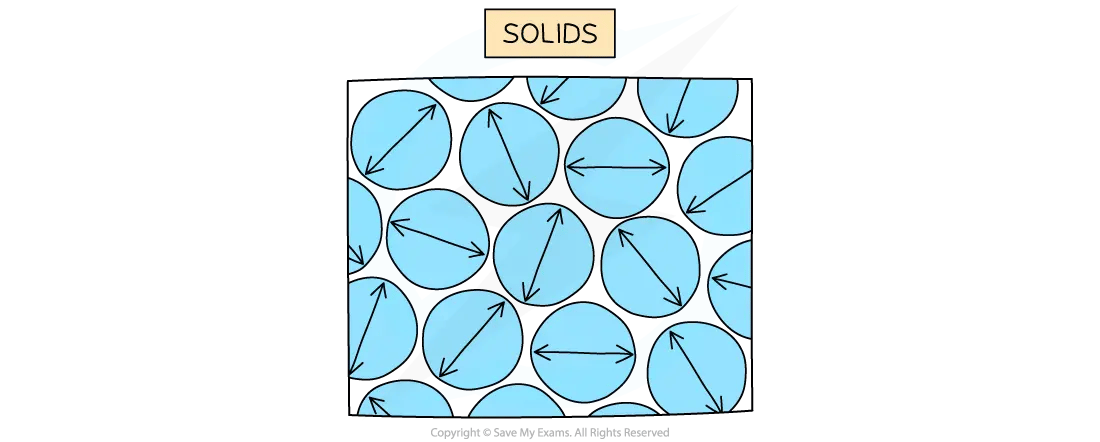
Solids
In a solid, the particles:
are closely packed together
vibrate about fixed positions
The particles in a solid have the least kinetic energy
Therefore, the particles cannot move very much
The particles are bound by the intermolecular forces of attraction
The density of particles in a solid is high
The particles are tightly packed together
Solids have:
a definite shape (they are rigid)
a definite volume
Particle arrangement in solids
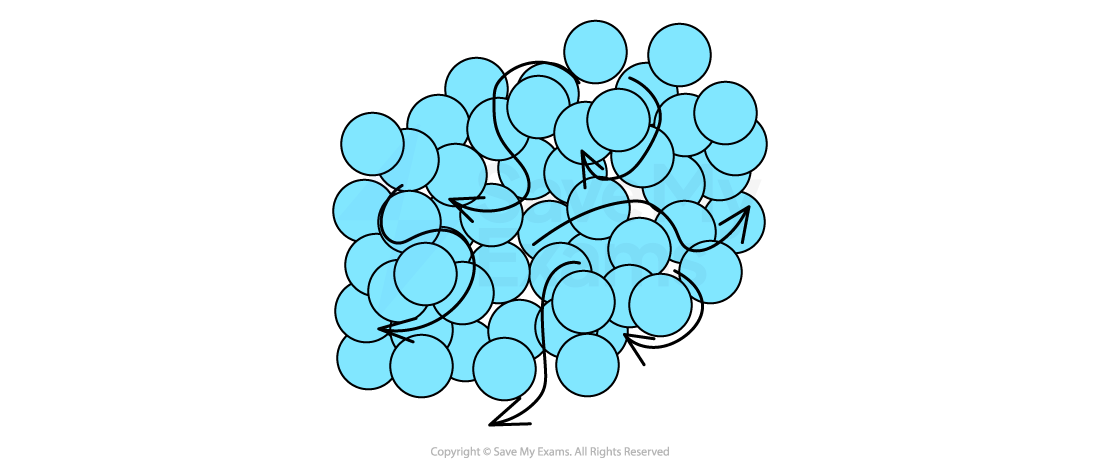
Liquids
In a liquid, the particles:
are closely packed together
can flow over one another
The particles in a liquid have more kinetic energy than the particles in a solid
Therefore, they can flow
The particles have enough energy to partially overcome the intermolecular forces of attraction
The density of particles in a liquid is medium
There is generally more space between the particles than in a solid
Liquids have:
no definite shape – they flow and will take the shape of their container
a definite volume
Particle arrangement in liquids
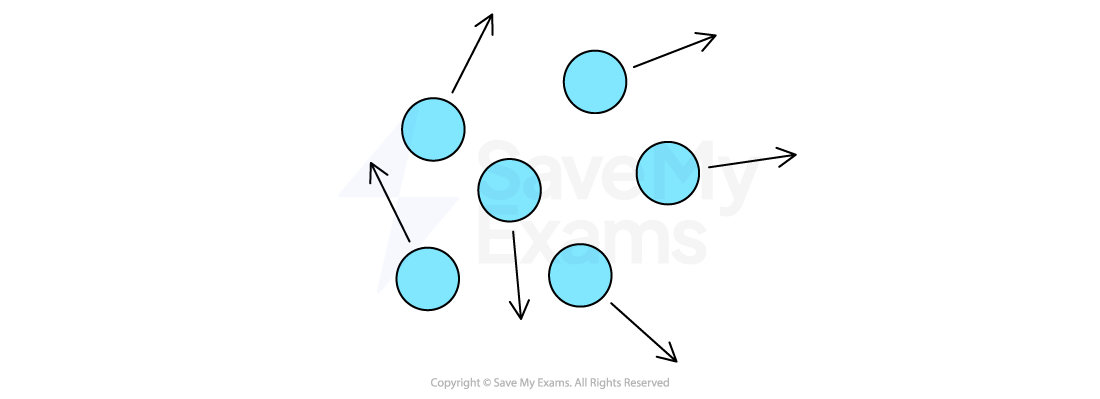
Gases
In a gas, the particles:
are far apart
move randomly
The particles in a gas have a lot of kinetic energy
Therefore, they are constantly moving and colliding with each other and the container walls
The particles have enough energy to overcome the intermolecular forces of attraction
Gases have:
no definite shape – they will take the shape of their container
no fixed volume – if placed in an evacuated container, they will expand to fill the container
The density of particles in a gas is low
There is a lot of space between the particles
Gases are highly compressible because:
there are large gaps between the particles
it is easier to push the particles closer together than in solids or liquids
Particle arrangement in gases
Solids, liquids and gases

Solid, Liquid, Gas Summary Table
State | Solid | Liquid | Gas |
|---|---|---|---|
Density | High | Medium | Low |
Arrangement of Particles | Regular | Random | Random |
Movement of Particles | Vibrate around a fixed position | Move around each other | Move quickly in all directions |
Energy of Particles | Low energy | Greater energy | Highest energy |
Examiner Tips and Tricks
Remember that the strength of the intermolecular forces of attraction are different for different substances, but for a given substance, they are the same for all states of matter. It is the energy of the particles that changes (with temperature) which determines which state of matter the substance is in.

Unlock more, it's free!
Did this page help you?