The Mass of Atoms (Oxford AQA IGCSE Combined Science Double Award): Revision Note
Exam code: 9204
Relative Masses
Protons, neutrons and electrons are so small that it is not practical to measure their mass using conventional units, such as grams
Instead, their masses are compared to each other
This is why they are called relative masses
Protons and neutrons have a very similar mass
So, they are both assigned a relative mass of 1
Electrons are roughly 2000 times smaller than a proton and neutron
So, the mass of an electron is described as very small or negligible
The relative masses of the subatomic particles are:
Table of relative masses
Sub-atomic particle | Relative mass |
|---|---|
Proton | 1 |
Neutron | 1 |
Electron | very small |
Atomic Number & Mass Number
Atomic Number
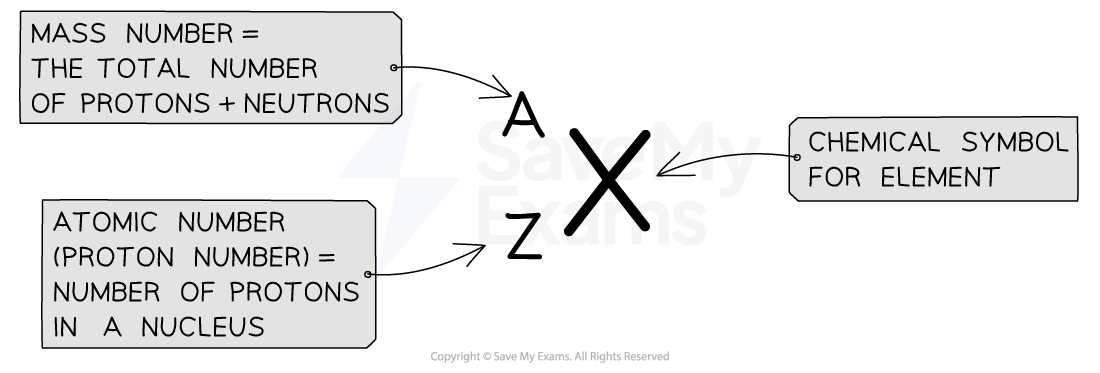
The atomic number (or proton number) is the number of protons in the nucleus of an atom
The symbol for this number is Z
The atomic / proton number is unique to each element, so no two elements have the same number of protons
Mass Number
The mass number is the total number of protons and neutrons in the nucleus of an atom
The symbol for this number is A
Representing Atoms
Every element is shown on the periodic table
Each element has its own symbol, mass number and atomic number and is represented as shown
Atomic Number & Mass Number diagram
Examiner Tips and Tricks
Both the atomic number and the mass number are given on the periodic table, but it can be easy to confuse them.
Think MASS = MASSIVE, as the mass number is always the big number, the small number is therefore the atomic number.
Worked Example
An element of sodium is shown on the periodic table as:
For an atom of sodium, state the following:
The number of protons
The number of protons and neutrons
Answer:
An atom of sodium contains:
11 protons
23 protons and neutrons
Using the information from a chemical symbol, it is possible to calculate the number of protons, neutrons and electrons

Unlock more, it's free!
Did this page help you?