Decay Equations (Edexcel IGCSE Physics (Modular)) : Revision Note
Alpha, beta, gamma & neutron emission
Alpha decay
During alpha decay, an alpha particle is emitted from an unstable nucleus
A completely new element is formed in the process
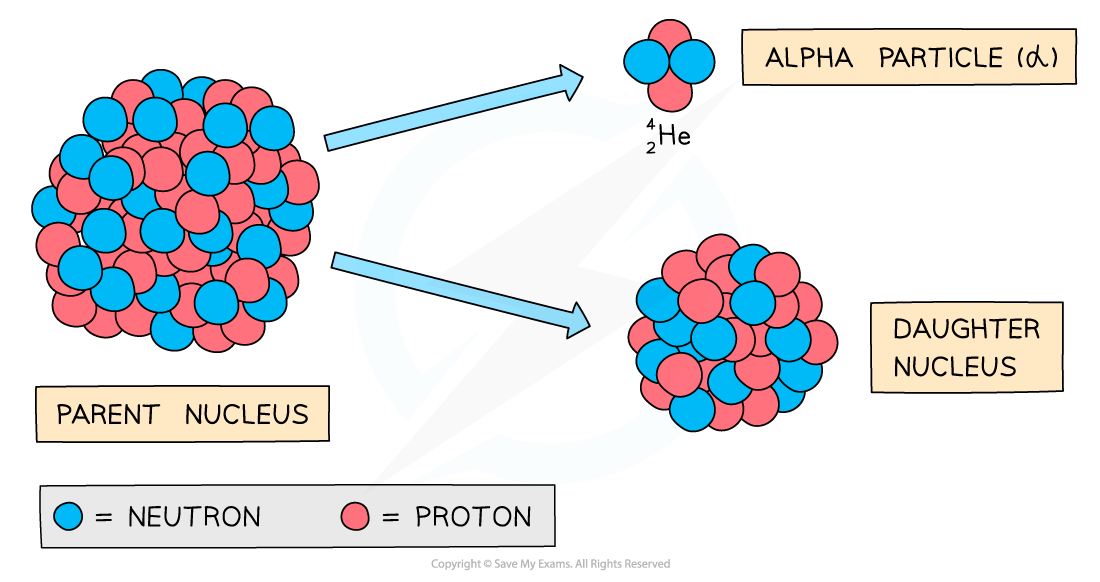
Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease
An alpha particle is a helium nucleus
It is made of 2 protons and 2 neutrons
When the alpha particle is emitted from the unstable nucleus, the mass number and atomic number of the nucleus changes
The mass number decreases by 4
The atomic number decreases by 2
Alpha decay can be represented by the following nuclear equation:
Where:
is the initial element X with mass number A and atomic number Z
is the new element Y
is an alpha particle
Beta decay
During beta decay, a neutron changes into a proton and an electron
The electron is emitted and the proton remains in the nuclei
A completely new element is formed because the atomic number changes

Beta decay often happens in unstable nuclei that have too many neutrons. The mass number stays the same, but the atomic number increases by one
A beta particle is a high-speed electron
It has a mass number of 0
This is because the electron has a negligible mass, compared to neutrons and protons
Therefore, the mass number of the decaying nuclei remains the same
Electrons have an atomic number of -1
This means that the atomic number of the new nucleus will increase by 1 to balance the overall atomic number before and after the decay
Beta decay can be represented by the following nuclear equation:
Where:
is the initial element X with mass number A and atomic number Z
is the new element Y
is a beta particle
Gamma decay
During gamma decay, a gamma ray is emitted from an unstable nucleus
The process that makes the nucleus less energetic but does not change its structure

Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus
The gamma ray that is emitted has a lot of energy, but no mass or charge
Gamma decay can be represented by the following nuclear equation:
Where:
is the element X with mass number A and atomic number Z
is a gamma ray
Notice that the mass number and atomic number of the unstable nucleus remains the same during the decay
Neutron emission
A small number of isotopes can decay by emitting neutrons
When a nucleus emits a neutron:
The atomic number (number of protons) does not change
The mass number (total number of nucleons) decreases by 1
Neutron emission can be represented by the following nuclear equation:
Where:
is the element X with mass number A and atomic number Z
is a neutron
Notice that the atomic number remains the same during the decay but the mass number has changed
This means an isotope of the original element has formed
Examiner Tips and Tricks
It is easy to forget that an alpha particle is a helium nucleus. The two are interchangeable, so don’t be surprised to see either used in the exam.
You are not expected to know the names of the elements produced during radioactive decays, but you do need to be able to calculate the mass and atomic numbers by making sure they are balanced on either side of the reaction.
Decay equations
Radioactive decay events can be shown using nuclear decay equations
A decay equation is similar to a chemical reaction equation as
the particles present before the decay are shown before the arrow
the particles produced in the decay are shown after the arrow
In a decay equation:
the sum of the mass numbers before and after the reaction must be the same
the sum of the atomic numbers before and after the reaction must be the same
The following decay equation shows polonium-212 undergoing alpha decay
When a nucleus of polonium-212 decays, a nucleus of lead-208 forms and an alpha particle is emitted
To check if the equation is balanced:
mass number: 212 = 208 + 4
atomic number: 84 = 82 + 2
The sum of the numbers are the same on each side, so the equation is balanced
Worked Example
A nucleus with 84 protons and 126 neutrons undergoes alpha decay. It forms lead, which has the element symbol Pb.
A.
B.
C.
D.
Which of the isotopes of lead pictured is the correct one formed during the decay?
ANSWER: A
Step 1: Calculate the mass number of the original nucleus
The mass number is equal to the number of protons plus the number of neutrons
The original nucleus has 84 protons and 126 neutrons
84 + 126 = 210
The mass number of the original nucleus is 210
Step 2: Calculate the new atomic number
The alpha particle emitted is made of two protons and two neutrons
Protons have an atomic number of 1, and neutrons have an atomic number of 0
Removing two protons and two neutrons will reduce the atomic number by 2
84 – 2 = 82
The new nucleus has an atomic number of 82
Step 3: Calculate the new mass number
Protons and neutrons both have a mass number of 1
Removing two protons and two neutrons will reduce the mass number by 4
210 – 4 = 206
The new nucleus has a mass number of 206
Worked Example
A nucleus with 11 protons and 13 neutrons undergoes beta decay. It forms magnesium, which has the element symbol Mg.
A.
B.
C.
D.
Which is the correct isotope of magnesium formed during the decay?
ANSWER: D
Step 1: Calculate the mass number of the original nucleus
The mass number is equal to the number of protons plus the number of neutrons
The original nucleus has 11 protons and 13 neutrons
11 + 13 = 24
The mass number of the original nucleus is 24
Step 2: Calculate the new atomic number
During beta decay a neutron changes into a proton and an electron
The electron is emitted as a beta particle
The neutron has an atomic number of 0 and the proton has an atomic number of 1
So the atomic number increases by 1
11 + 1 = 12
The new nucleus has an atomic number of 12
Step 3: Calculate the new mass number
Protons and neutrons both have a mass number of 1
Changing a neutron to a proton will not affect the mass number
The new nucleus has a mass number of 24 (the same as before)

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?


