Half-Life (Cambridge (CIE) IGCSE Co-ordinated Sciences (Double Award)): Revision Note
Exam code: 0654 & 0973
Did this video help you?
Half-life
The half-life of a particular isotope is defined as:
The time taken for half the nuclei of that isotope in any sample to decay
The rate at which the activity of a sample decreases is measured in terms of half-life
This is the time it takes for the activity of a sample to fall to half its original level
This is the time it takes for the activity of the sample to decrease from 100 % to 50 %
It is the same length of time as it would take to decrease from 50 % activity to 25 % activity
Different isotopes have different half-lives and half-lives can vary from a fraction of a second to billions of years in length
The half-life is constant for a particular isotope
Representing half life
Half-life can be determined from an activity–time graph
A half-life graph
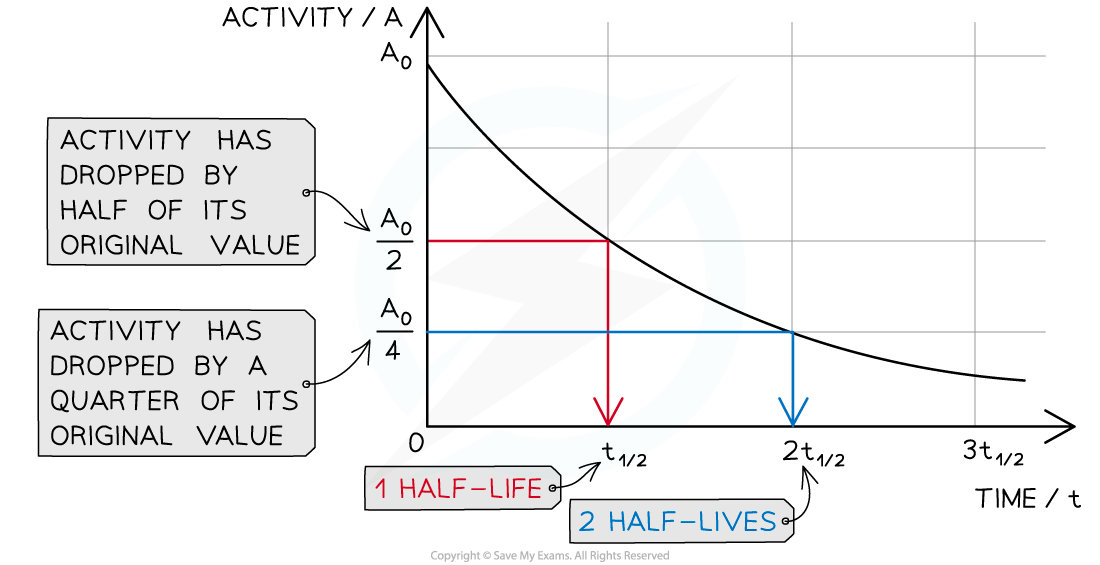
The graph shows how the activity of a radioactive sample changes over time. Each time the original activity halves, another half-life has passed
Half-life can also be represented on a table
As the number of the half-life increases, the proportion of the isotope remaining halves
Table showing the number of half-lives to the proportion of isotope remaining
Number of half-lives | Proportion of isotope remaining |
|---|---|
0 | 1 |
1 | |
2 | |
3 | |
4 | |
... | ... |
Worked Example
An isotope of protactinium-234 has a half-life of 1.17 minutes.
Calculate the amount of time it takes for a sample to decay from a mass of 10 mg to 2.5 mg.
Answer:
Step 1: Calculate the fraction of the sample remaining
Initial mass of sample = 10 mg
Final mass of sample = 2.5 mg
The fraction of the sample remaining is
Step 2: Calculate the number of half-lives that have passed
Using the table above we can see that two half-lives have passed
Step 3: Calculate the time for the sample to decay
Two half lives have passed
So the time for the sample to decay is twice the half-life
The time for the sample to decay to a mass of 2.5 mg is 2.34 minutes
Did this video help you?

Unlock more, it's free!
Did this page help you?