Temperature (Edexcel IGCSE Physics (Modular)): Revision Note
Exam code: 4XPH1
Temperature & speed
Imagine molecules of gas that are free to move around in a box
The molecules in the gas move around randomly at high speeds, colliding with surfaces and exerting pressure upon them
The temperature of a gas is a measure of the average speed of the molecules:
the higher the temperature of the gas, the faster the molecules move
This is because they have a greater average speed
Gas molecules move about randomly at high speeds
Temperature & kinetic energy
Heating a system will change the energy stored in a system by increasing the kinetic energy of its particles
The Kelvin temperature of the gas is related to the average kinetic energy of the molecules
This increase in kinetic energy (and therefore energy stored in the system) can:
Cause the temperature of the system to increase
Or, produce a change of state (solid to liquid or liquid to gas)
The internal energy of a gas is the sum of the kinetic energy of all the molecules
The higher the temperature, the higher the average kinetic energy of the molecules and vice versa
This means they move around faster
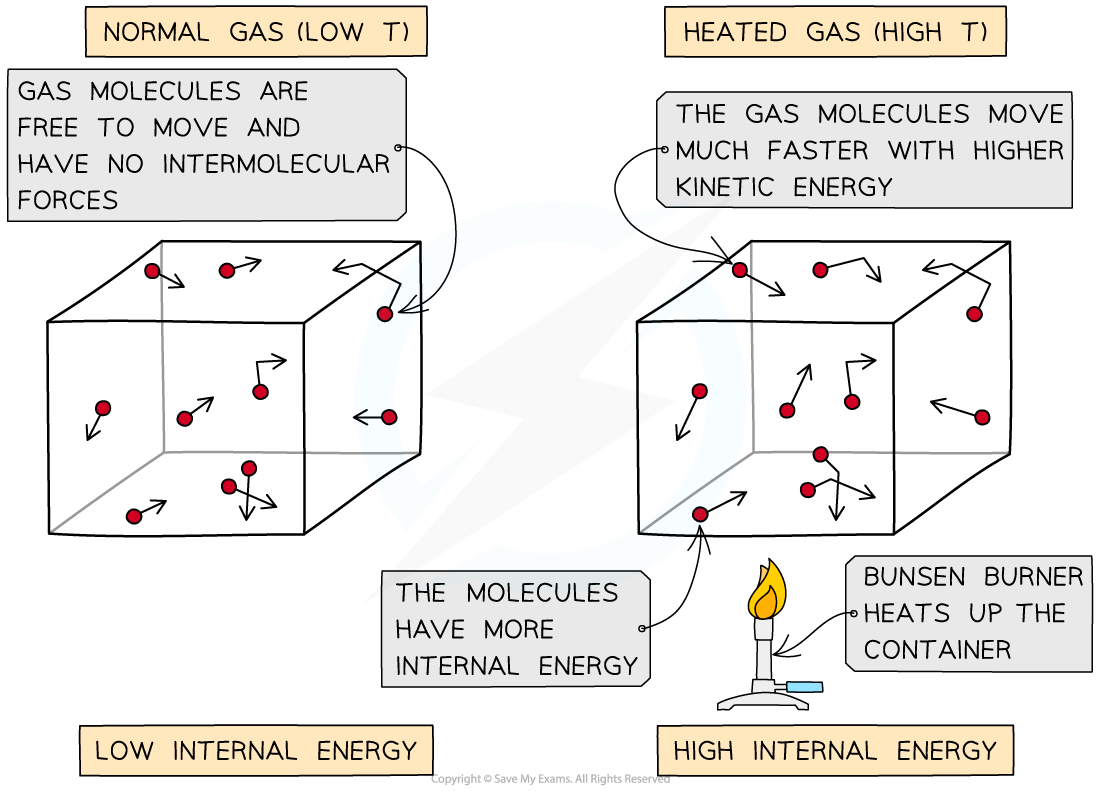
As the container is heated up, the gas molecules move faster with higher kinetic energy. The energy stored within the system - the internal energy - therefore increases
If the temperature of a gas is increased, the particles move faster and gain kinetic energy
Therefore, they will collide more with each other and the container,, leading to an increase in pressure
The temperature (in Kelvin) is proportional to the average kinetic energy of the molecules
T ∝ KE
Worked Example
When a liquid evaporates, higher-energy molecules escape from the surface of the liquid. Which row best describes what happens to the temperature of the liquid and the average kinetic energy of the molecules within it?
| Temperature / K | Average kinetic energy of the molecules |
A | Increases | Increases |
B | Decreases | Decreases |
C | Stays the same | Stays the same |
D | Decreases | Increases |
ANSWER: B
When evaporation takes place, the more energetic molecules are leaving the surface of the liquid
Since the more energetic molecules have left, the average kinetic energy per molecule must decrease
Therefore, A, C & D are not correct
Temperature is proportional to the average kinetic energy per molecule, therefore the temperature also decreases

Unlock more, it's free!
Did this page help you?