Atomic Structure (Edexcel IGCSE Physics) : Revision Note
Learn about atomic structure for your IGCSE Physics exam. This revision note includes sub-atomic particles and thier charge, atomic numbers and mass numbers.
Did this video help you?
Atomic structure
Atoms are the building blocks of all matter
They are incredibly small, with a radius of only 1 × 10-10 m
This means that about one hundred million atoms could fit side by side across your thumbnail
Atoms have a tiny, dense nucleus at their centre, with electrons orbiting around the nucleus
The radius of the nucleus is over 10,000 times smaller than the whole atom, but it contains almost all of the mass of the atom
Atomic structure of lithium
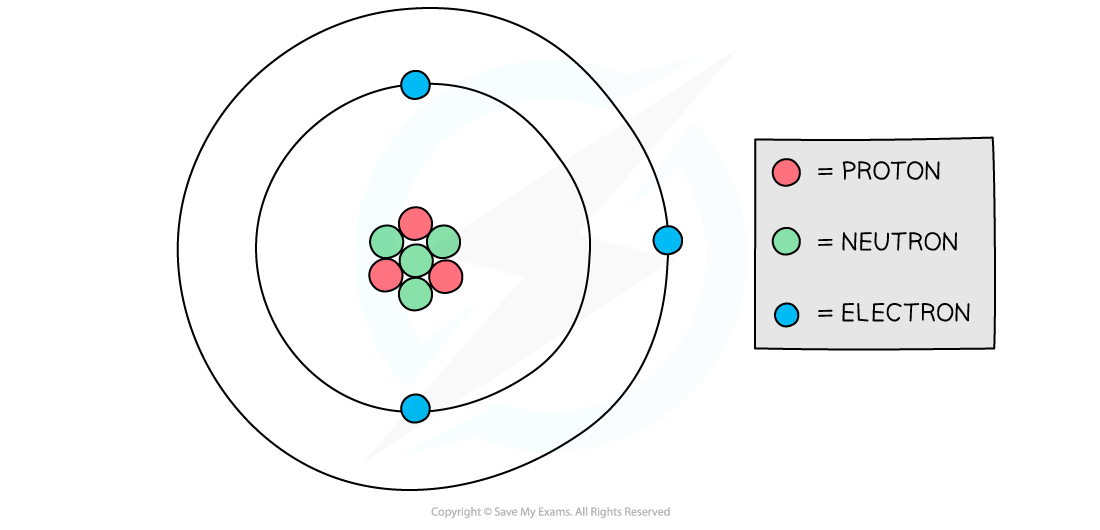
Diagram showing the structure of a Lithium atom. If drawn to scale then the electrons would be around 100 metres away from the nucleus!
Particles in the atom
The nucleus contains:
Protons - positively charged particles with a relative atomic mass of one unit
Neutrons – no charge, and also with a relative atomic mass of one unit
Almost all of the atom is empty space, but moving around the nucleus there are:
Electrons – negative charge with almost no mass (1/2000 the mass of a proton or neutron)
The properties of each of the particles are shown in the table below:
Table of particle properties
Particle | Location | Relative charge | Relative mass |
|---|---|---|---|
proton | in the nucleus | +1 | 1 |
neutron | in the nucleus | 0 | 1 |
electron | orbiting the nucleus | −1 | 1/2000 (negligible) |
Charge in the atom
Although atoms contain particles of different charge, the total charge within an atom is zero
This is because the number of electrons is equal to the number of protons
The following table sets out the calculation of the total charge in the lithium atom in the diagram above:
Calculating total charge table
Particle | Relative charge | Number of particles in lithium atom | number × relative charge | Total charge |
|---|---|---|---|---|
proton | +1 | 3 | +3 | (+3) + 0 + (−3) = 0 |
neutron | 0 | 4 | 0 | |
electron | −1 | 3 | −3 |
If an atom loses electrons, then it is said to be ionised
Symbols are used to describe particular nuclear by their element symbol, atomic number and mass number
This notation is called nuclear notation

Carbon 12 in nuclear notation
Worked Example
A nucleus of carbon-12 is shown below.

How many electrons are there in an atom of carbon-12?
Answer:
Step 1: Count the number of protons in the carbon nucleus
There are 6 protons in the carbon atom
Step 2: Determine the number of electrons
Remember, the number of electrons in an atom is equal to the number of protons
Therefore there must be 6 electrons in the carbon atom
Examiner Tips and Tricks
You may have noticed that the number of electrons is not part of the mass number. This is because electrons have a tiny mass compared to neutrons and protons. We say their mass is negligible when compared to the particles in the nucleus.
Did this video help you?
Atomic & mass number
Atomic number
The number of protons in an atom is called its atomic number (it can also be called the proton number)
Elements in the periodic table are ordered by their atomic number
Therefore, the number of protons determines which element an atom is
The atomic number of a particular element is always the same
For example:
Hydrogen has an atomic number of 1. It always has just one proton
Sodium has an atomic number of 11. It has 11 protons
Uranium has an atomic number of 92. It has 92 protons
The atomic number is also equal to the number of electrons in an atom
This is because atoms have the same number of electrons and protons in order to have no overall charge
Mass number
The total number of particles in the nucleus of an atom is called its mass number (it can also be called the nucleon number)
The mass number is the number of protons and neutrons in the atom
The number of neutrons can be found by subtracting the atomic number from the mass number
number of neutrons = mass number – atomic number
For example, if a sodium atom has a mass number of 23 and an atomic number of 11, then the number of neutrons would be 23 – 11 = 12
Nuclear notation
The mass number and atomic number of an atom are shown by writing them with the atomic symbol
This is called nuclear notation
Here are three examples:

Examples of nuclear notation for atoms of Hydrogen, Sodium and Uranium
The top number is the mass number
This is equal to the total number of particles (protons and neutrons) in the nucleus
The lower number is the atomic number
This is equal to the total number of protons in the nucleus
The atomic and mass number of each type of atom in the examples above is shown in this table:
Number of protons, neutrons & electrons table
Atom | Number of protons | Number of neutrons | Number of electrons |
|---|---|---|---|
hydrogen | 1 | 1 | 1 |
sodium | 11 | 12 | 11 |
uranium | 92 | 143 | 92 |
Worked Example
The element symbol for gold is .
How many protons, neutrons and electrons are in an atom of gold?
| number of protons | number of neutrons | number of electrons |
|---|---|---|---|
A. | 79 | 79 | 79 |
B. | 197 | 79 | 118 |
C. | 118 | 118 | 79 |
D. | 79 | 118 | 79 |
ANSWER: D
Step 1: Determine the atomic and mass number
The gold atom has an atomic number of 79 (lower number) and a mass number of 197 (top number)
Step 2: Determine the number of protons
The atomic number is equal to the number of protons
An atom of gold has 79 protons
Step 3: Calculate the number of neutrons
The mass number is equal to the number of protons and neutrons
The number of neutrons is equal to the mass number minus the atomic number
number of neutrons = 197 − 79 = 118
An atom of gold has 118 neutrons
Step 4: Determine the number of electrons
An atom has the same number of protons and electrons
An atom of gold has 79 electrons

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
