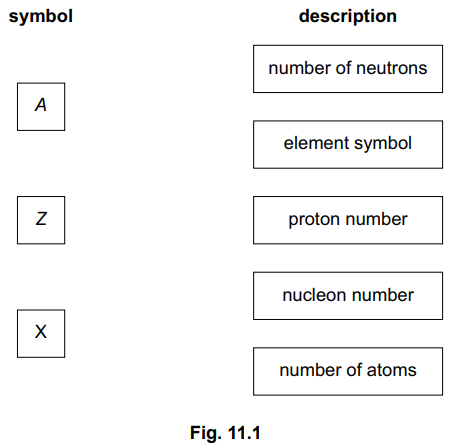
State the term used to describe nuclides which have the same number of protons but different numbers of neutrons.
Table 1.1 describes four nuclides. The nuclide notation for lead-206 is missing.
name of nuclide | radium-222 | radon-222 | lead-216 | lead-206 |
nuclide notation |
|
Table 1.1
(i) State which two nuclides have the same number of protons.
[1]
(ii) Complete the table by filling in the nuclide notation for lead-206.
[1]
Using the information from Table 1.1
(i) State which two nuclides have the same number of nucleons.
[1]
(ii) State which two nuclides have the same number of neutrons.
[1]
(iii) State which one of the four nuclides has the most electrons orbiting when it is in a neutral atom.
[1]
Was this exam question helpful?