Radioactive Decay (Cambridge (CIE) IGCSE Physics): Revision Note
Exam code: 0625 & 0972
Did this video help you?
Effect of nuclear size on decay
Extended tier only
Isotopes of an element may be radioactive due to an excess of neutrons in the nucleus and/or the nucleus being too heavy
Excess of neutrons
The most stable nuclei have roughly the same number of protons as neutrons
Too many protons in a nucleus means the repulsive force between them is large, causing the neutrons to repel each other
So, a nucleus with an imbalance of protons or neutrons is more likely to decay into several smaller nuclei until stable nuclei are obtained
With roughly the same number of nucleons in each nucleus
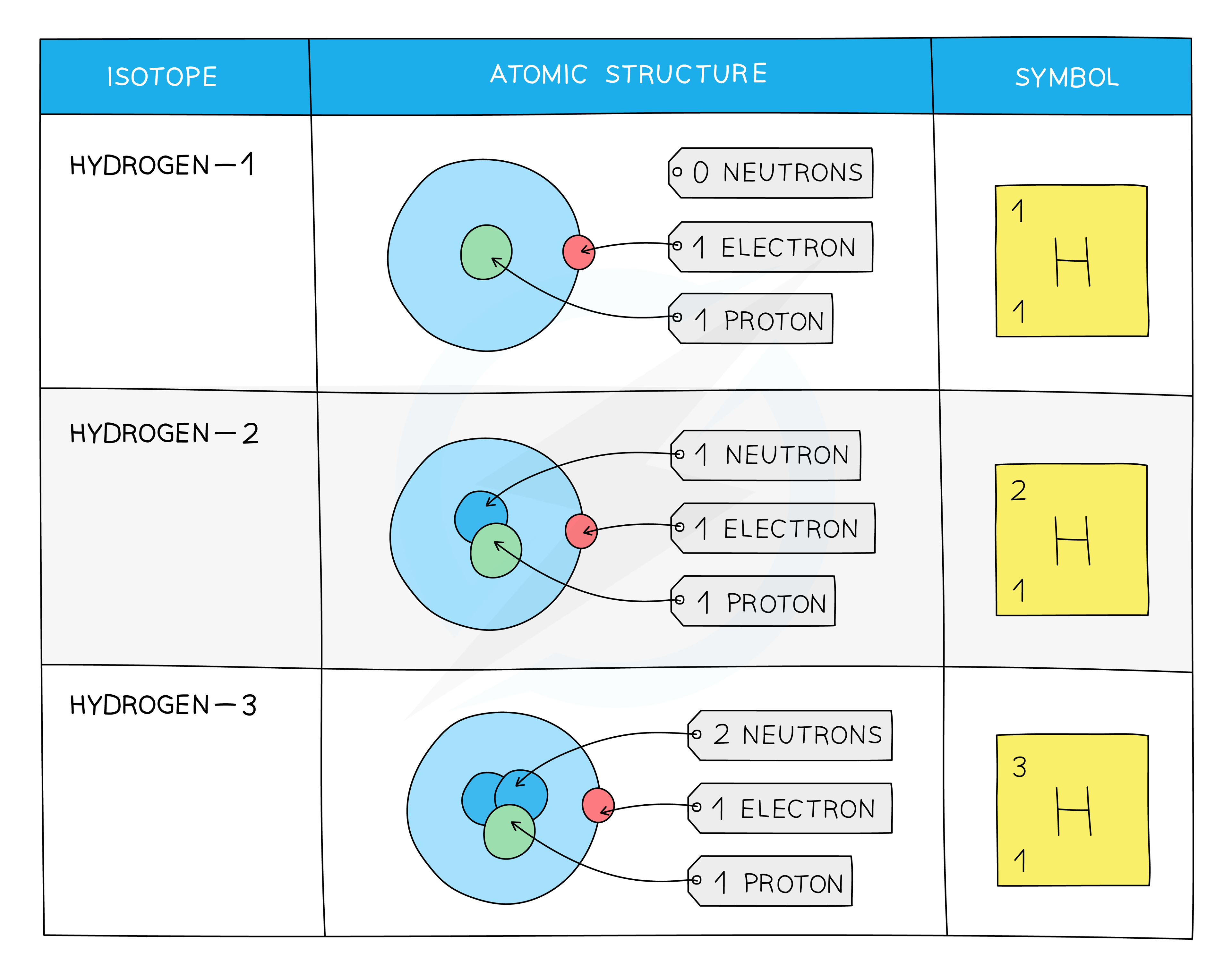
An example of this is the isotope of hydrogen–1
H-1 is the stable nucleus of hydrogen with 0 neutrons and 1 proton
H-2 (deuterium) has one more neutron in the nucleus
H-3 (tritium) has 2 neutrons to 1 proton. This is much more unstable than H-1 or H-2
Hydrogen isotopes
Heavy nucleus
If a nucleus is too heavy, this means it has too many protons and neutrons
The forces keeping the protons and neutrons together in the nucleus will be weaker
An example of this is uranium–238
It has a nucleus with 238 protons and neutrons
During nuclear decay, the mass number of the element which it decays into is gradually reduced
This is done through alpha (α) or beta (β) decay
Uranium–238 decay chain
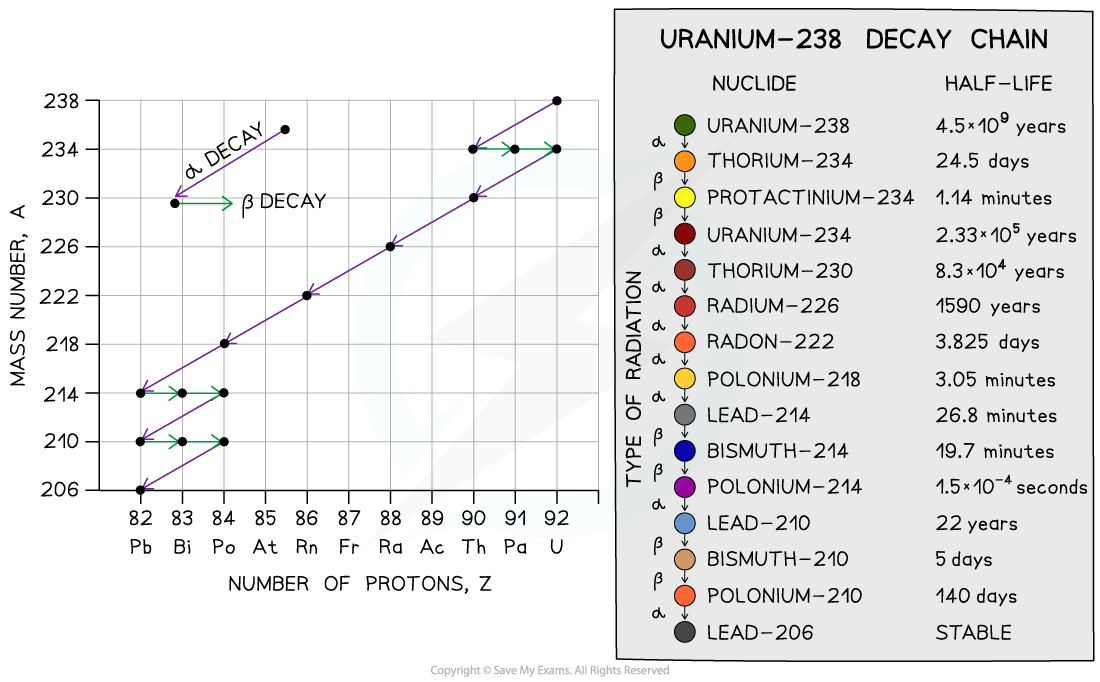
The graph shows the decay chain of uranium-238 through alpha and beta emission
Examiner Tips and Tricks
The notation of C-12 for example, means the element 'carbon' with the mass (or nucleon) number of 12.
Change to a new element
A nucleus changes to a different element, during α-decay or β-decay
The initial nucleus is often called the parent nucleus
The nucleus of the new element produced is often called the daughter nucleus
The daughter nucleus is a new element because it has a different proton and/or nucleon number than the original parent nucleus
This can be seen on a graph of N (neutron number) against Z (proton number)
For example; when Pu-239 decays by alpha to U-235, it loses 2 protons and 2 neutrons
U (uranium) is a completely different element from Pu (plutonium)
Graph of neutron number against proton number

Graph of N against Z for the decay of Pu–239
Reducing neutron number
Extended tier only
α and β-decay affect the nucleus by
increasing its stability
reducing the number of excess neutrons
Alpha decay
During alpha decay an alpha particle is emitted from an unstable nucleus
A completely new element is formed in the process
Alpha decay
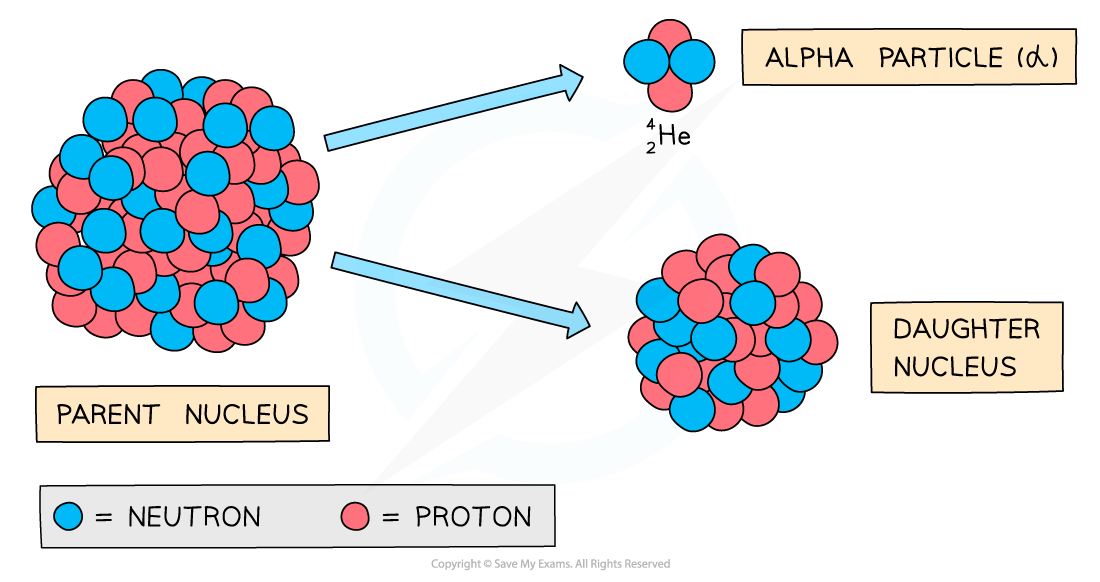
Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease
An alpha particle is a helium nucleus
It is made of 2 protons and 2 neutrons
When the alpha particle is emitted from the unstable nucleus, the mass number and atomic number of the nucleus changes
The mass number decreases by 4
The atomic number decreases by 2
The charge on the nucleus also decreases by 2
This is because protons have a charge of +1 each
Beta decay
During beta decay, a neutron changes into a proton and an electron
The electron is emitted and the proton remains in the nucleus
A completely new element is formed because the atomic number changes
Beta decay

Beta decay often happens in unstable nuclei that have too many neutrons. The mass number stays the same, but the atomic number increases by one
A beta particle is a high-speed electron
It has a mass number of 0
This is because the electron has a negligible mass, compared to neutrons and protons
Therefore, the mass number of the decaying nucleus remains the same
Electrons have an atomic number of -1
This means that the new nuclei will increase their atomic number by 1 so atomic number is conserved before and after the decay
Gamma decay
During gamma decay, a gamma ray is emitted from an unstable nucleus
This process makes the nucleus less energetic but does not change its structure because gamma radiation has no mass or charge
Gamma decay

Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus
Examiner Tips and Tricks
There is a second form of beta decay during which a proton changes into a neutron. This is called beta-plus decay - you might come across it while revising, but you don't need to know about it for your exam. Only use the information here for your iGCSE.
It is easy to forget that an alpha particle is a helium nucleus, or that a beta particle is an electron. Look out for either wording!
Did this video help you?
Decay equations
Extended tier only
Decay equations, use nuclide notation, to show the emission of α-particles, β-particles and γ-radiation
A decay equation is similar to a chemical reaction equation
The particles present before the decay are shown before the arrow
The particles produced in the decay are shown after the arrow
During decay equations, the sum of the mass and atomic numbers before the reaction must be the same as the sum of the mass and atomic numbers after the reaction
Alpha decay equation
All alpha decay equations have the following form for isotopes X and Y:
The following decay equation shows polonium-212 undergoing alpha decay
It forms lead-208 and an alpha particle
An alpha particle can also be written as a helium (He) nucleus
Beta decay equation
All beta decay equations have the following form for isotopes X and Y:
Gamma decay equation
All gamma decay equations have the following form for isotope X
Worked Example
A nucleus with 84 protons and 126 neutrons undergoes alpha decay. It forms lead, which has the element symbol Pb.
A
B
C
D
Which isotope of lead pictured is the correct one formed during the decay?
Answer: A
Step 1: Calculate the mass number of the original nucleus
The mass number is equal to the number of protons plus the number of neutrons
The original nucleus has 84 protons and 126 neutrons
The mass number of the original nucleus is 210
Step 2: Calculate the new atomic number
The alpha particle emitted is made of two protons and two neutrons
Protons have an atomic number of 1, and neutrons have an atomic number of 0
Removing two protons and two neutrons will reduce the atomic number by 2
The new nucleus has an atomic number of 82
Step 3: Calculate the new mass number
Protons and neutrons both have a mass number of 1
Removing two protons and two neutrons will reduce the mass number by 4
The new nucleus has a mass number of 206
Worked Example
A nucleus with 11 protons and 13 neutrons undergoes beta decay. It forms magnesium, which has the element symbol Mg.
A
B
C
D
Which is the correct isotope of magnesium formed during the decay?
Answer: D
Step 1: Calculate the mass number of the original nucleus
The mass number is equal to the number of protons plus the number of neutrons
The original nucleus has 11 protons and 13 neutrons
The mass number of the original nucleus is 24
Step 2: Calculate the new atomic number
During beta decay a neutron changes into a proton and an electron
The electron is emitted as a beta particle
The neutron has an atomic number of 0 and the proton has an atomic number of 1
So the atomic number increases by 1
The new nucleus has an atomic number of 12
Step 3: Calculate the new mass number
Protons and neutrons both have a mass number of 1
Changing a neutron to a proton will not affect the mass number
The new nucleus has a mass number of 24 (the same as before)
Examiner Tips and Tricks
You are not expected to know the names of the elements produced during radioactive decays, but you do need to be able to calculate the mass and atomic numbers by making sure they are balanced on either side of the reaction.
Did this video help you?

Unlock more, it's free!
Did this page help you?