Fission & Fusion (Cambridge (CIE) IGCSE Physics): Revision Note
Exam code: 0625 & 0972
Did this video help you?
Fission & fusion
Nuclear fission & fusion are nuclear reactions that change the nucleus of an atom to produce high amounts of energy from the energy stored in the nucleus of an atom
Nuclear fission
Nuclear fission is defined as:
The splitting of a large, unstable nucleus into two smaller nuclei
During fission:
A neutron collides with an unstable nucleus
The neutron and the nucleus are the reactants
The nucleus splits into two smaller nuclei (called daughter nuclei) and two or three neutrons
The daughter nuclei and the neutrons are the products of the reaction
Gamma rays are also emitted
Nuclear fission process
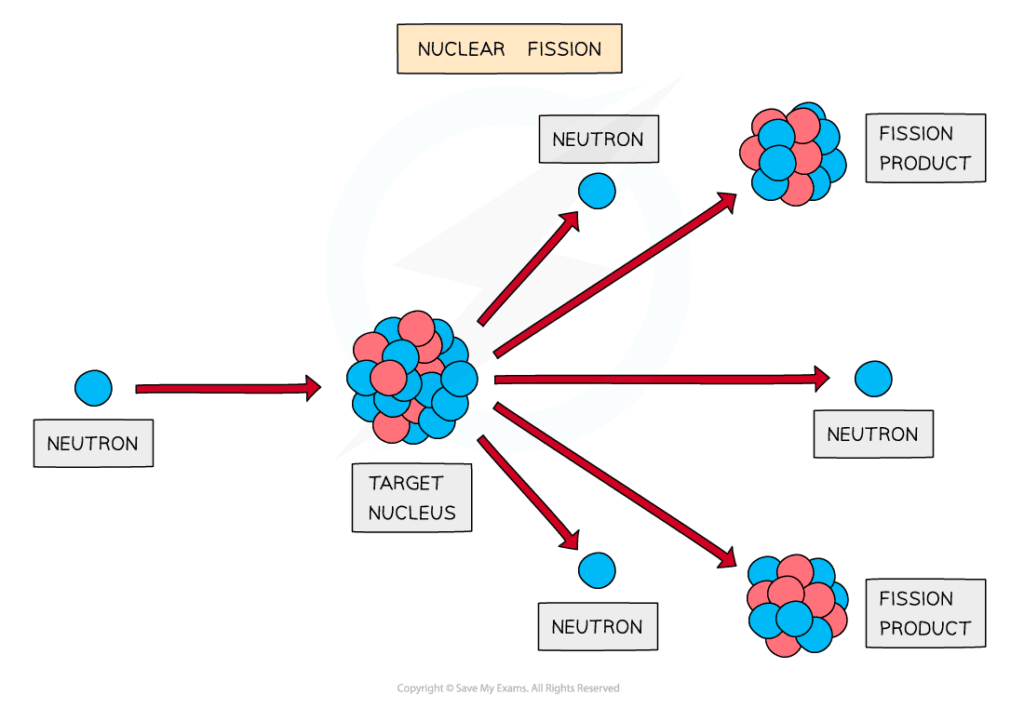
A neutron is fired into the target nucleus, causing it to split
Nuclear fission nuclide equations
An example of a nuclide equation for the fission of uranium-235 is:
Where:
is an unstable isotope of uranium
is a neutron
is an unstable isotope of krypton
is an unstable isotope of barium
Nuclear fission of uranium-235

Large nuclei can decay by fission to produce smaller nuclei and neutrons with a lot of kinetic energy
Nuclear fission mass and energy values
Energy is conserved in a nuclear fission reaction
In the example:
The sum of the nucleon (top) numbers of the reactants (left-hand side) is equal to the sum of the nucleon numbers of the products (right-hand side):
The same is true for the proton (bottom) numbers:
The products of fission move away very quickly
During a fission reaction, energy is transferred from nuclear energy store of the parent nucleus to the kinetic energy store of the products
The mass of the products is less than the mass of the original nucleus
This is because the remaining mass has been converted into energy, which is released during the fission process
Large isotopes with a large nucleon number, such as uranium and plutonium, both undergo fission and are used as fuels in nuclear power stations
Nuclear fusion
Nuclear fusion is defined as:
When two light nuclei join to form a heavier nucleus
Stars use nuclear fusion to produce energy
In most stars, hydrogen nuclei (light nuclei) are fused together to form a helium nucleus (heavier nucleus) and massive amounts of energy is produced
Nuclear fusion of hydrogen

Two hydrogen nuclei fuse to form a helium nucleus
Nuclear fusion requires extremely high temperature and pressure
So fusion is very hard to reproduce on Earth
Nuclear fusion nuclide equations
An example of a nuclide equation for fusion is:
Where:
is deuterium (isotope of hydrogen with 1 proton and 1 neutron)
is hydrogen (with one proton)
is an isotope of helium (with two protons and one neutron)
Nuclear fusion mass and energy values
The energy produced during nuclear fusion comes from a very small amount of a particle’s mass converted into energy
Therefore, the mass of the product (fused nucleus) is less than the mass of the two original nuclei (reactants)
The remaining mass has been converted into the energy released when the nuclei fuse
The amount of energy released during nuclear fusion is huge:
The energy from 1 kg of hydrogen that undergoes fusion is equivalent to the energy from burning about 10 million kilograms of coal
Worked Example
A nuclide equation for nuclear fission is stated as:
Calculate the number of neutrons, N emitted in this reaction.
Answer:
Step 1: Calculate the sum of the nucleon numbers of the reactants
The reactants are on the left-hand side of the equation
The nucleon numbers are the top numbers in the nuclide notation
235 + 1 = 236
Step 2: Calculate the sum of the nucleon numbers of the products
The products are on the right-hand side of the equation
96 + 137 + (N × 1) = 233 + N
Step 3: Equate the total nucleons of the reactants and products
236 = 233 + N
Step 4: Rearrange for the number of neutrons, N
N = 236 – 233 = 3

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?