The Nucleus (Cambridge (CIE) IGCSE Physics) : Revision Note
Did this video help you?
Composition of the nucleus
A nucleus is composed of:
positively charged protons
neutrally charged neutrons
Hence a nucleus has an overall positive charge
Structure of the atom

Protons and neutrons are found in the nucleus of an atom
Examiner Tips and Tricks
Be careful with your terminology:
Atom = nucleus (proton and neutron) and electrons
Nucleus = protons and neutrons at the centre of the atom
Did this video help you?
Describing the nucleus
Proton number, Z
The number of protons in an atom is called its proton number (it can also be called the atomic number)
Elements in the periodic table are ordered by their atomic number
Therefore, the number of protons determines which element an atom is
The atomic number of a particular element is always the same
For example:
Hydrogen has an atomic number of 1. It always has just one proton
Sodium has an atomic number of 11. It has 11 protons
Uranium has an atomic number of 92. It has 92 protons
The atomic number is also equal to the number of electrons in an atom
This is because atoms have the same number of electrons and protons in order to have no overall charge
Nucleon number, A
The total number of particles in the nucleus of an atom is called its nucleon number (or mass number)
The mass number is the number of protons and neutrons in the atom
Calculating the number of neutrons
The number of neutrons can be found by subtracting the atomic number from the mass number
For example, if a sodium atom has a mass number of 23 and an atomic number of 11, then the number of neutrons would be 23 – 11 = 12
Examiner Tips and Tricks
You may have noticed that the number of electrons is not part of the mass number. This is because electrons have a tiny mass compared to neutrons and protons. We say their mass is negligible when compared to the particles in the nucleus.
Nuclide notation
Atomic symbols are written in a specific notation called ZXA or nuclide notation
The top number A represents the nucleon number
The lower number Z represents the proton number
Nuclide notation
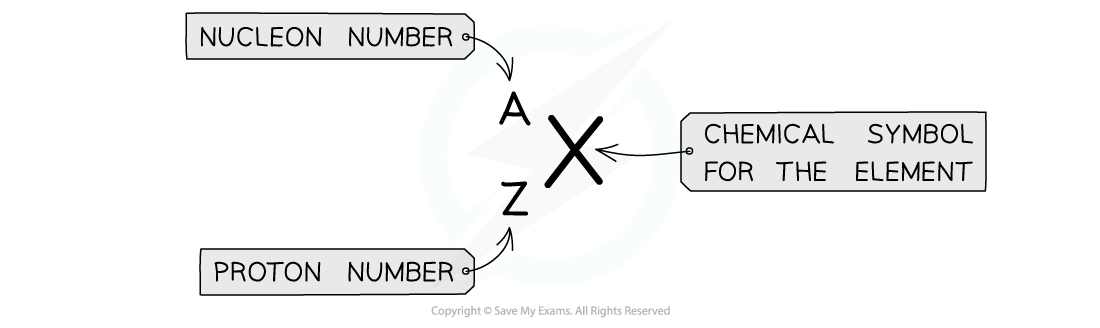
Atomic symbols in AZX nuclide notation describe the constituents of nuclei
A nuclide is a group of atoms containing the same number of protons and neutrons
For example, 5 atoms of oxygen are all the same nuclide but are 5 separate atoms
An example of nuclide notation is:
Example of lithium nuclide notation

Atomic symbols, like the one above, describe the constituents of nuclei
Worked Example
The element symbol for gold is Au. How many protons, neutrons and electrons are in the gold atom?
| Protons | Neutrons | Electrons |
|---|---|---|---|
A | 79 | 79 | 79 |
B | 197 | 79 | 118 |
C | 118 | 118 | 79 |
D | 79 | 118 | 79 |
Answer: D
Step 1: Determine the atomic and mass number
The gold atom has an atomic number of 79 (lower number) and a mass number of 197 (top number)
Step 2: Determine the number of protons
The atomic number is equal to the number of protons
The atom has 79 protons
Step 3: Calculate the number of neutrons
The mass number is equal to the number of protons and neutrons
The number of neutrons is equal to the mass number minus the atomic number
The atom has 118 neutrons
Step 4: Determine the number of electrons
An atom has the same number of protons and electrons
The atom has 79 electrons
Examiner Tips and Tricks
You may recognise this notation from the periodic table in chemistry when mass number and proton number are more commonly used. In physics, you are more likely to see nucleon number and proton number. The periodic table is ordered by atomic number.
Did this video help you?
Isotopes
Although the number of protons in a particular element is always the same, the number of neutrons can be different
Isotopes are atoms of the same element that have an equal number of protons but a different number of neutrons
This means that each element can have more than one isotope
Isotopes tend to be more unstable due to their imbalance of protons and neutrons
This means they're more likely to decay
In the diagram below are three isotopes of Hydrogen:
Isotopes of hydrogen
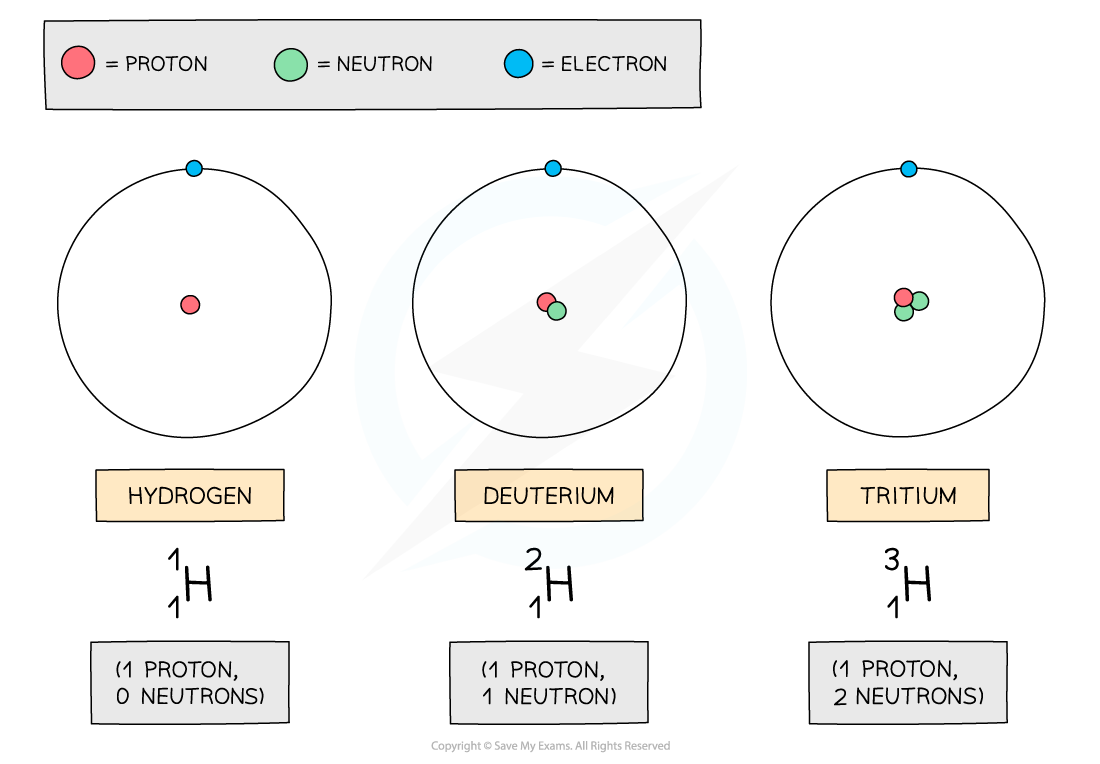
Hydrogen has three isotopes, each with a different number of neutrons
Isotopes occur naturally, but some are more rare than others
For example, about 2 in every 10,000 Hydrogen atoms is Deuterium
Tritium is even more rare (about 1 in every billion billion hydrogen atoms)
Worked Example
Which of the following elements are isotopes of each other?
A |
|
B |
|
C |
|
D |
|
Answer: B
In nuclide notion, the top number is the nucleon number (number of protons and neutrons) and the bottom number is the proton number (number of protons)
Isotopes are two of the same elements
This eliminates option D since one is oxygen (O) and the other nitrogen (N)
Which have the same number of protons
This eliminates option C and A
Their proton numbers are different for the same element
But a different number of neutrons
Therefore, the correct answer is B

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?

