Specific Heat Capacity (Cambridge (CIE) IGCSE Physics): Revision Note
Exam code: 0625 & 0972
Did this video help you?
Internal energy
A rise in the temperature of an object increases its internal energy
This can be thought of as due to an increase in the average speed of the particles
Increasing speed increases kinetic energy
Internal energy is defined as:
The total energy stored inside a system by the particles that make up the system due to their motion and positions
Motion of the particles affects their kinetic energy
Positions of the particles relative to each other affects their potential energy
Together, these two make up the internal energy of the system

Substances have internal energy due to the motion of the particles and their positions relative to each other
Average kinetic energy
Extended tier only
Heating a system changes a substance's internal energy by increasing the kinetic energy of its particles
The temperature of the material, therefore, is related to the average kinetic energy of the molecules
An increase in temperature leads to an increase of the average kinetic energy of the particles in the substance
This also means that internal energy increases, as internal energy is the sum of all kinetic and potential energies
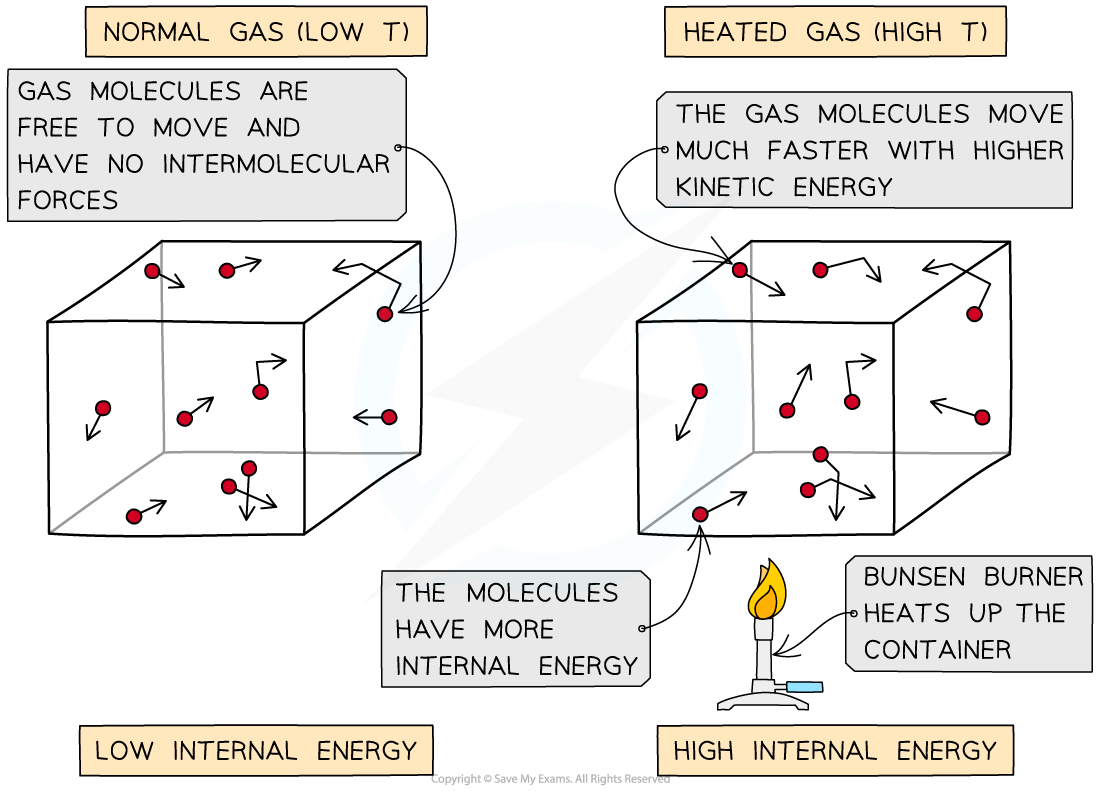
As the container heats up, the gas molecules move faster. Faster motion causes higher kinetic energy and therefore higher internal energy
Worked Example
What property of an object is a measure of the energy in the kinetic stores of its particles?
Answer:
As temperature increases, so does the average kinetic energy of the particles
This means temperature is a measure of the energy in the kinetic stores of the particles
Specific heat capacity
Extended tier only
What is specific heat capacity?
When heated, a substance's temperature can increase
The amount by which the temperature increases depends on:
The mass of the substance heated
The type of material
The amount of thermal energy transferred in to the system
The specific heat capacity, c, of a substance is defined as:
The energy required per unit mass per unit temperature increase
In other words, this is the amount of energy required to raise the temperature of 1 kg of a substance by 1 °C
Different substances have different specific heat capacities
If a substance has a low specific heat capacity, it heats up and cools down quickly (i.e. it takes less energy to change its temperature)
If a substance has a high specific heat capacity, it heats up and cools down slowly (i.e. it takes more energy to change its temperature)
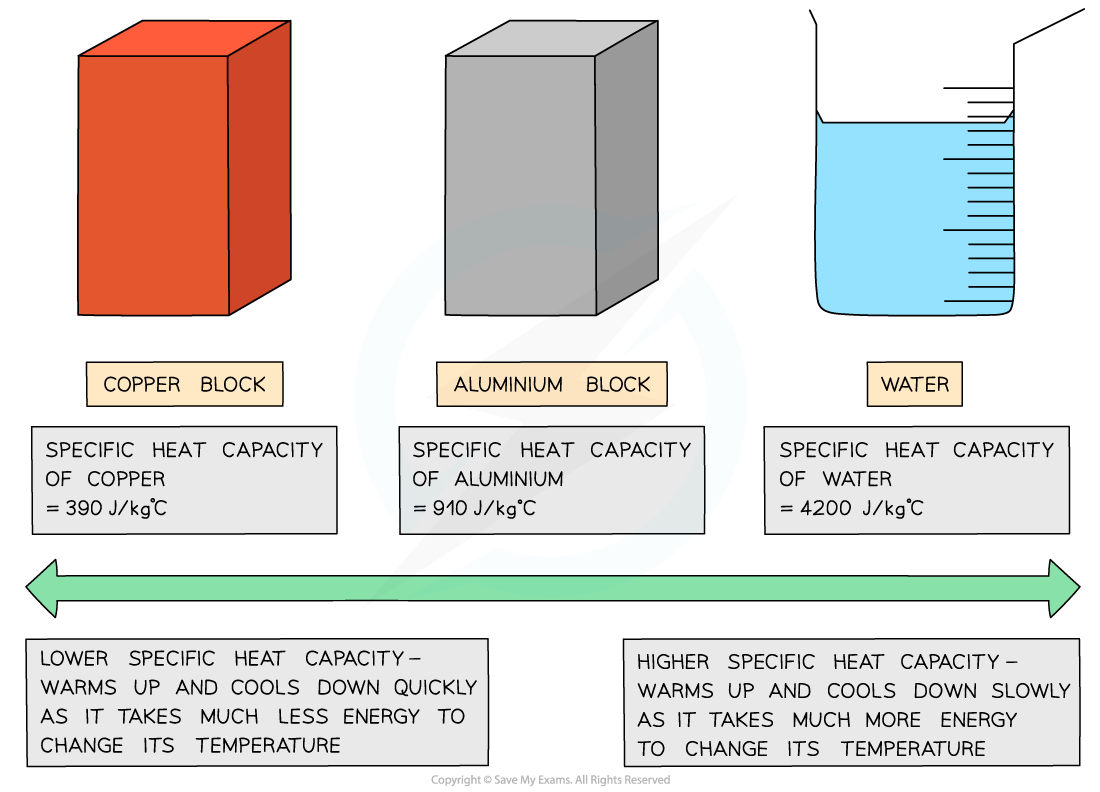
Low vs high specific heat capacity
Calculating specific heat capacity
The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation:
Where:
ΔE = change in thermal energy, in joules (J)
m = mass, in kilograms (kg)
c = specific heat capacity, in joules per kilogram per degree Celsius (J/kg °C)
Δθ = change in temperature, in degrees Celsius (°C)
Worked Example
What unit is used to measure energy when calculating specific heat capacity?
A. kilograms
B. joules
C. joules per kilogram per degree Celsius
D. kelvin
Answer: B
The question asks what unit is used to measure energy when calculating specific heat capacity
The units of energy are joules
A common mistake is for students to see 'specific heat capacity' and state the units of that, not energy
Reading the question thoroughly and underlining key information avoids these mistakes
Worked Example
Water of mass 0.48 kg is increased in temperature by 0.7 °C. The specific heat capacity of water is 4200 J / kg °C.
Calculate the amount of thermal energy transferred to the water.
Answer:
Step 1: Write down the known quantities
Mass, m = 0.48 kg
Change in temperature, ΔT = 0.7 °C
Specific heat capacity, c = 4200 J/kg °C
Step 2: Write down the relevant equation
Step 3: Calculate the thermal energy transferred by substituting in the values
Step 4: Round the answer to 2 significant figures and include the units
ΔE = 1400 J
Examiner Tips and Tricks
While you must remember the equation for specific heat capacity, you will always be given the specific heat capacity of a substance so you do not need to memorise any values.
However, it's useful to have the general idea that, the larger the number, the less the substance will increase in temperature for a given amount of heat.
You can see this for yourself in your own kitchen at home. Metal pans, which have a relatively low specific heat capacity get very hot, very quickly when put on the hob. Add water to the pan, which has a relatively high specific heat capacity and the water will take much longer to heat up.
Notice the units of specific heat capacity:
joules per kilogram per degree Celsius : J / kg °C
'per' means 'divided by'. We say 'per' in front of every value that is being divided by, hence 'per kilogram per degree Celsius'

Unlock more, it's free!
Did this page help you?