Pressure & Temperature in Gases (Cambridge (CIE) IGCSE Co-ordinated Sciences (Double Award)): Revision Note
Exam code: 0654 & 0973
Pressure & temperature in gases
A change in temperature or pressure affects the volume of gases
As the air inside a hot air balloon is heated up, it expands and the balloon gets bigger
This is because the volume of a gas increases as temperature increases

As temperature increases gas volume increases. The density decreases as the volume increases so the balloon rises.
If you have a gas stored inside a container that is squeezed, the pressure increases as you decrease the volume
This is what happens in a bicycle pump

Pressure increases as volume decreases in a bicycle pump
As you compress the bicycle pump, the high pressure allows you to inflate a tyre
You can feel the force of the high pressure if you put your finger on the end of the pump
Gases & kinetic theory
Extended tier only
Gaseous particles are in constant and random motion
The pressure that a gas creates inside a closed container is produced by the gaseous particles hitting the inside walls of the container:

Moving particles of gas colliding with each other and the container walls
How does temperature affect the volume of a gas?
Increasing the temperature increases the kinetic energy of each particle
Remember: The thermal energy from increasing the temperature is converted to kinetic energy in the particles
As the temperature increases, the particles in the gas move faster and spread out more
If the gas particles are inside a container, they will collide with the container walls more frequently
If the container walls are flexible and stretchy then the container will get bigger and bigger, just like the hot air balloon!
Examiner Tips and Tricks
If you are talking about the particles, then make sure that you talk about them spreading out.
If you are talking about the material, then you can use the word expand.
You will lose a mark in an exam if you talk about particles expanding!
How does pressure affect the volume of a gas?
Pressure is about the number of particles in a given volume
Increasing the pressure means that there are the same number of particles but in a smaller volume
Conversely, decreasing the pressure means that there are the same number of particles but in a larger volume
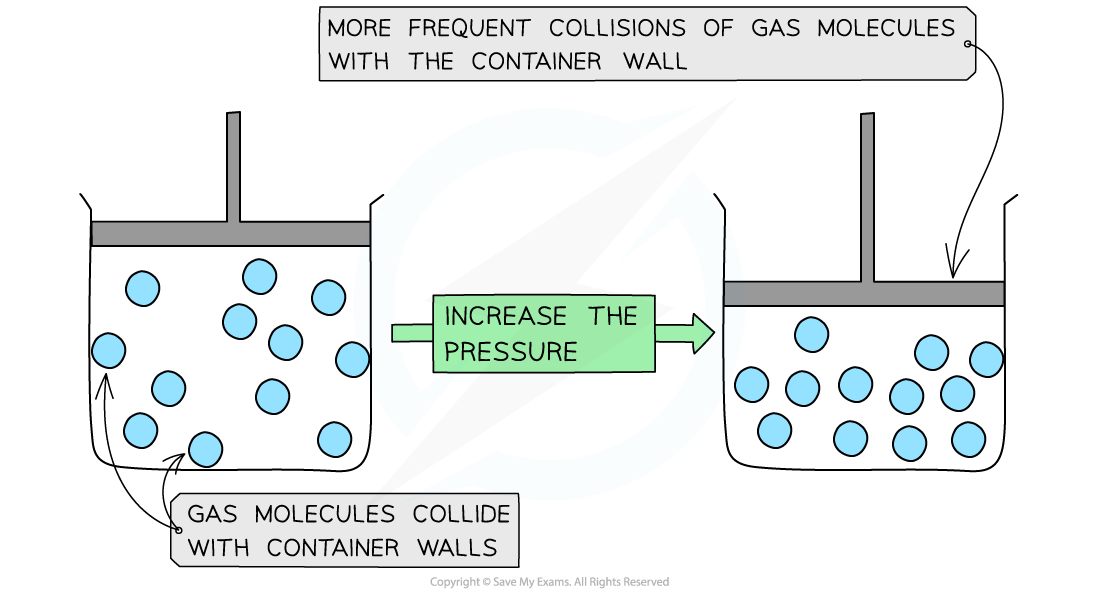
When the pressure increases, the volume decreases. This means that the molecules collide with the container walls more frequently
Since the volume is decreased, the gas particles hit the container walls more frequently
If the pressure is too high, this can result in the container leaking gas or exploding

Unlock more, it's free!
Did this page help you?