Endothermic & Exothermic Reactions (Cambridge (CIE) IGCSE Co-ordinated Sciences (Double Award)): Revision Note
Exam code: 0654 & 0973
Did this video help you?
Exothermic & endothermic reactions
Heat exchange in reactions
Chemical reactions occur so that elements can achieve a more stable energy state by gaining a full outer shell of electrons
This is done by chemical bonding
This process involves the transfer of thermal energy into and out of reaction mixtures
The terms used to describe this are:
System: the reacting chemicals
Surroundings: anything other than the chemicals reacting
The energy within the system comes from the chemical bonds themselves which could be considered as tiny stores of chemical energy
Exothermic reactions
In exothermic reactions, thermal energy is transferred from the system to the surroundings
The energy of the system decreases, which means that the energy change is negative
The temperature of the surroundings increases because thermal energy is given out / released
The overall transfer is from the system to the surroundings

Diagram showing the transfer of heat energy outwards from an exothermic reaction
Typical examples of exothermic reactions include:
Combustion
Oxidation
Neutralisation
Hand warmers used in the wintertime are based on the release of heat from an exothermic reaction
Self-heating cans of food and drinks such as coffee and hot chocolate also use exothermic reactions in the bases of the containers
Endothermic reactions
In endothermic reactions, thermal energy is transferred from the surroundings to the system
The energy of the system increases, which means that the energy change is positive
The temperature of the surroundings decreases because thermal energy is taken in / absorbed
The overall transfer is from the surroundings to the system
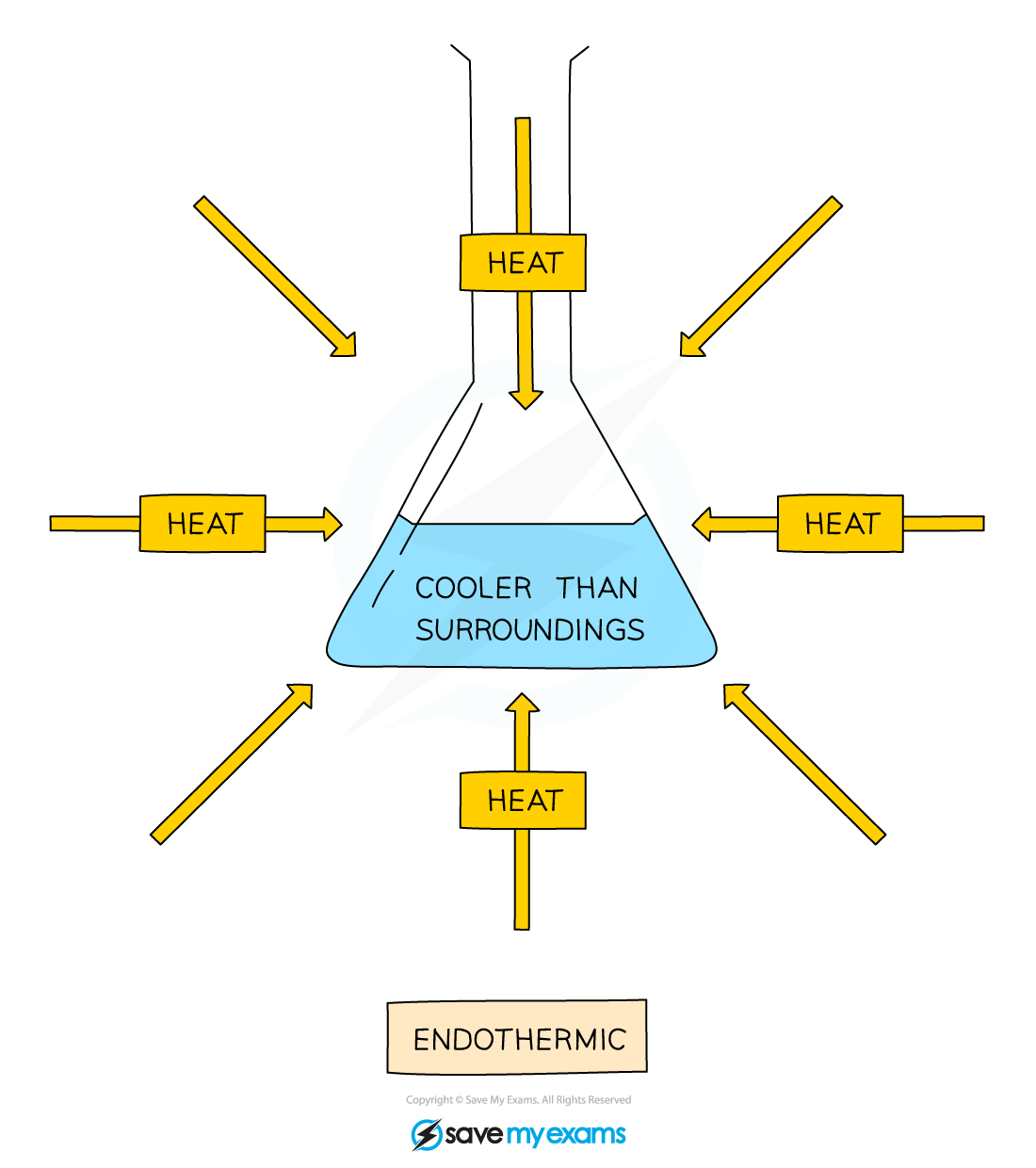
Diagram showing the transfer of heat energy from the surroundings into an endothermic reaction
Endothermic reactions are less common than exothermic reactions
Typical examples of endothermic reactions include:
Electrolysis
Thermal decomposition
The first stages of photosynthesis
Cold packs for sports injuries are based on endothermic reactions, designed to take heat away from a recently injured area to prevent swelling
Worked Example
A student was investigating the temperature change for four different chemical reactions. The table shows the chemicals that the student combined for each reaction along with the initial and final temperatures of the reaction.
Experiment | Chemicals | Initial temperature | Final temperature | |
1 | 10 cm3 NaOH | 10 cm3 HCl | 19 | 21 |
2 | 10 cm3 NaHCO3 | 2 g citric acid | 20 | 16 |
3 | 10 cm3 CuSO4 | 0.5 g Mg powder | 20 | 26 |
4 | 10 cm3 H2SO4 | 3 cm Mg ribbon | 19 | 31 |
Explain whether each reaction is endothermic or exothermic.
Answers:
Reactions 1, 3 and 4 are exothermic reactions because they show a temperature increase
Reaction 2 is an endothermic reaction because it shows a temperature decrease
Reaction pathway diagrams
Reaction pathway diagrams are graphical representations of the relative energies of the reactants and products in chemical reactions
On a reaction pathway diagram:
Progress of the reaction is shown on the x-axis
Energy is shown on the y-axis
The difference in height between the energy of reactants and products is the overall energy change of a reaction
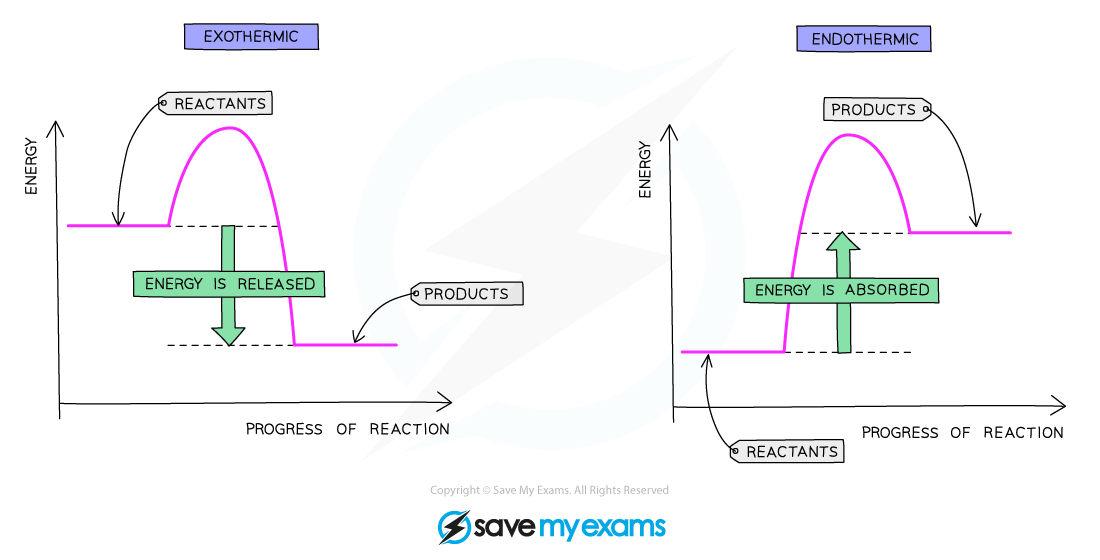
Reaction pathway diagram of an exothermic reaction and an endothermic reaction
In exothermic reactions:
Energy is given out to the surroundings
The energy of the products will therefore be lower than the energy of the reactants
The overall energy change is negative
This is represented on the reaction profile with a downwards-arrow as the energy of the products is lower than the reactants
In endothermic reactions:
Energy is taken in from the surroundings
The energy of the products will be higher than the energy of the reactants
The overall energy change is positive
This is represented on the reaction profile with an upwards-arrow as the energy of the products is higher than the reactants
Examiner Tips and Tricks
To help you remember whether a chemical system is exothermic or endothermic:
In EXothermic reactions heat Exits the system and in ENdothermic reactions heat ENters the system.
Exothermic reactions always give off heat and they feel hot
Endothermic reactions always take heat in and they feel cold.
Core candidates will be expected to interpret reaction pathway diagrams
Extended candidates will be expected to draw and interpret reaction pathway diagrams

Unlock more, it's free!
Did this page help you?